Perioperative: neoadjuvant KEYTRUDA plus chemotherapy followed by adjuvant KEYTRUDA monotherapy
Prescribing Information [External link]
The efficacy and safety of KEYTRUDA were investigated as perioperative treatment in early-stage NSCLC in…
KEYNOTE-671
…a Phase III, randomised, double-blind clinical study in 797 patients with resectable Stage II, IIIA or IIIB (N2)* NSCLC1
During this trial, KEYTRUDA achieved the dual primary endpoints of EFS and OS – see below for more details.
*Tumour staging system as per AJCC 8th edition.1
Licensed indication
KEYTRUDA, in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment, is indicated for the treatment of resectable non-small cell lung carcinoma at high risk of recurrence in adults.2
Key eligibility criteria:
- Pathologically confirmed, resectable Stage II, IIIA or IIIB (N2) NSCLC as per AJCC 8th edition
- ECOG PS 0 or 1
- Able to undergo surgery
- Provision of tumour sample for PD-L1 evaluation*
Key exclusion criteria:
- Prior therapy
- ILD or pneumonitis requiring steroids
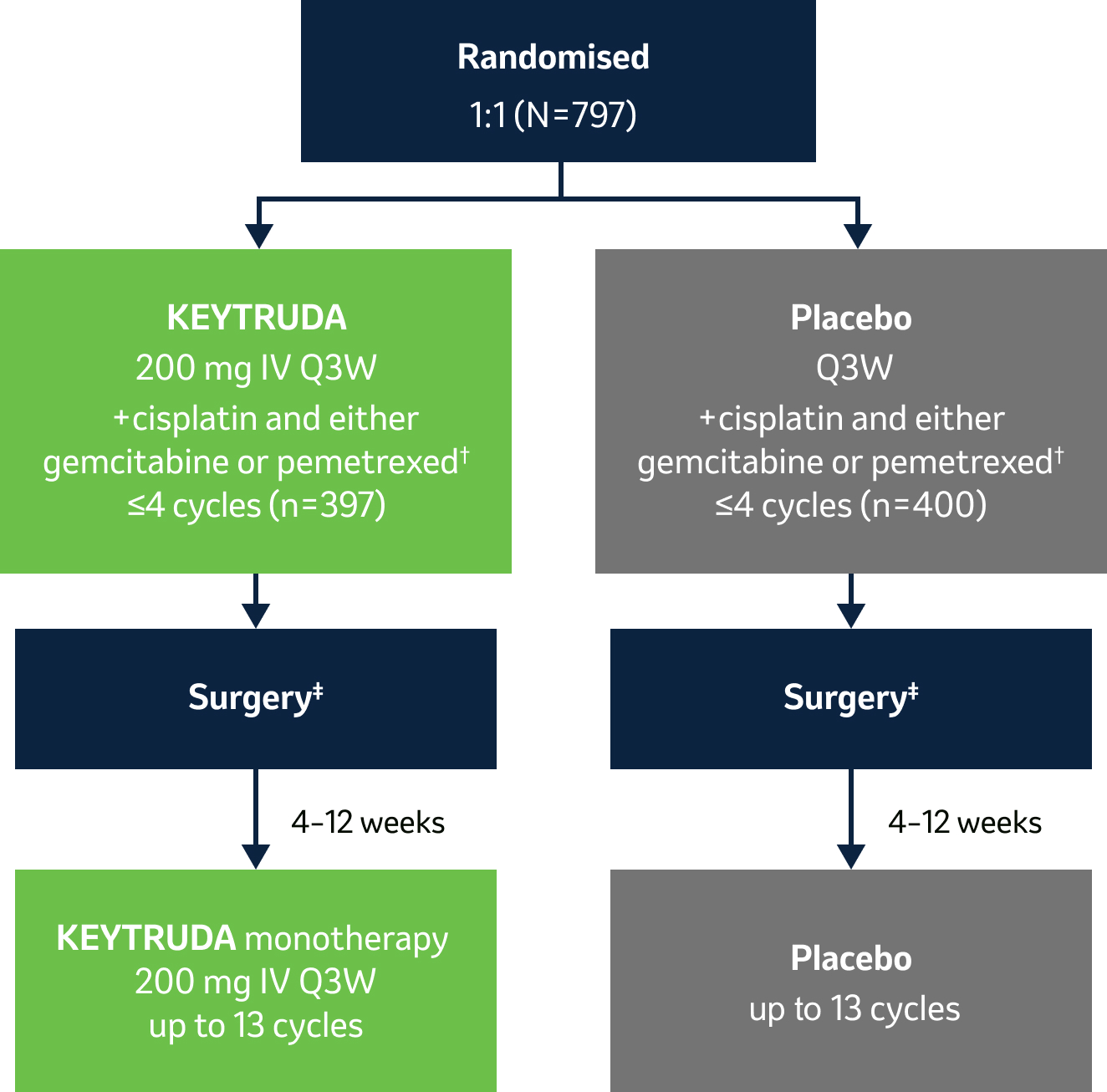
- Dual primary endpoints: EFS by investigator (as per RECIST v1.1) and OS§
- Secondary endpoints: mPR and pCR per blinded independent pathology review, and safety
Stratification factors:
- Stage (II vs III)
- PD-L1 TPS <50% vs ≥50%*
- Histology (squamous vs non-squamous)
- Region (East Asia vs other)
Surgical details||:
- KEYTRUDA arm: 92.0% (n=299/325) complete (R0) surgical resection; 78.8% lobectomies
- Placebo arm: 84.2% (n=267/317) complete (R0) surgical resection; 75.1% lobectomies
*PD-L1 TPS was assessed at a central laboratory using PD-L1 IHC 22C3 pharmDx.1
†Platinum-containing chemotherapy consisted of cisplatin (75 mg/m2 Q3W) combined with either gemcitabine (1000 mg/m2 on Days 1 and 8 Q3W; for squamous histology only) or pemetrexed (500 mg/m2 IV Q3W; for non-squamous histology only).1
‡Radiotherapy was to be administered to participants with microscopic positive margins, gross residual disease or extracapsular nodal extension following surgery, and to participants who did not undergo planned surgery for any reason other than local progression or metastatic disease.3
§One-sided alpha of 2.5% split between EFS and OS.1 EFS defined as the time from randomisation to the first occurrence of local progression that precluded the planned surgery, unresectable tumour, progression or recurrence according to RECIST v1.1, by the investigator’s assessment, or death from any cause and OS defined as the time from randomisation to death from any cause.1
¶mPR defined as ≤10% viable tumour cells in resected primary tumour and lymph nodes and pCR defined as the absence of residual invasive cancer in resected primary tumour and lymph nodes [ypT0/Tis ypN0]) as assessed on the basis of blinded, central examination by a pathologist.1
||IA1 dataset. Median follow-up was 25.2 months. Data cut-off date for IA1: 29 July 2022.1
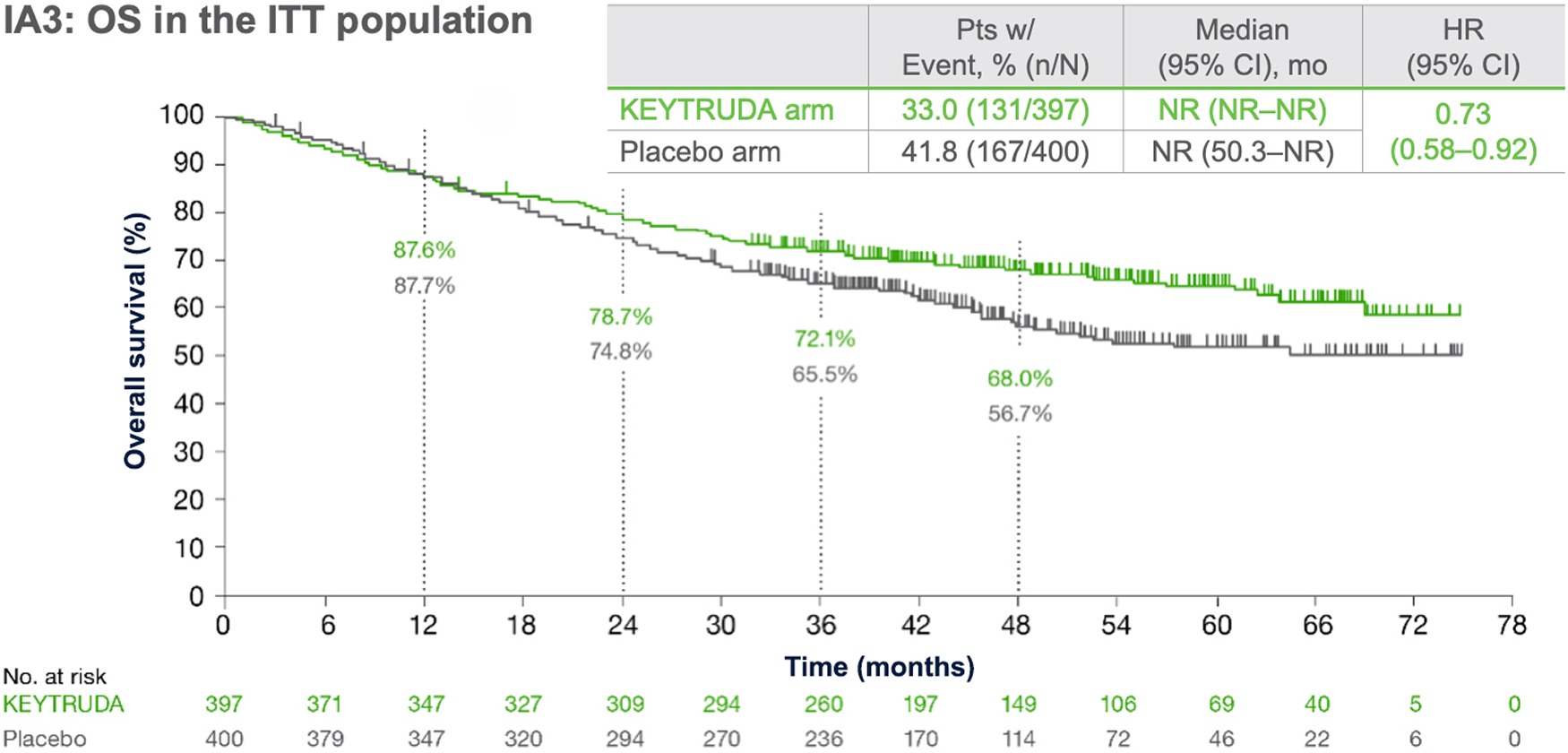
At the median follow-up of 36.6 months, perioperative KEYTRUDA demonstrated a statistically significant improvement in OS vs neoadjuvant Pt-CT and surgery alone4
KEYNOTE-671: OS in the ITT population (IA1, IA2 and IA3)*3–5
Dual primary endpoint
Median follow-up: 25.2 months (IA1); 36.6 months (IA2); 41.1 months (IA3)
Data cut-off date: 29 July 2022 (IA1); 10 July 2023 (IA2); 19 August 2024 (IA3)
- At IA1, OS was not mature, but showed a favourable trend (HR: 0.73)3
-
At IA2, the prespecified interim analysis for OS, a statistically significant and clinically meaningful OS benefit was shown with perioperative KEYTRUDA4
- OS HR was 0.72 (95% CI: 0.56–0.93); one-sided p=0.00517†4, representing a 28% reduction in risk of death, with the upper CI clearly below unity4
Adapted from Majem M, et al. 2024.5
- At IA3, perioperative KEYTRUDA continued to provide improved OS vs placebo5
Statistical significance for OS was demonstrated at IA2, therefore OS was not formally tested at IA3.3,5
*OS was defined as time from randomisation to death from any cause.3
†Significance boundary at IA2, one-sided p=0.00543.4
KEYNOTE-671 dual primary endpoint: OS in key ITT subgroups5
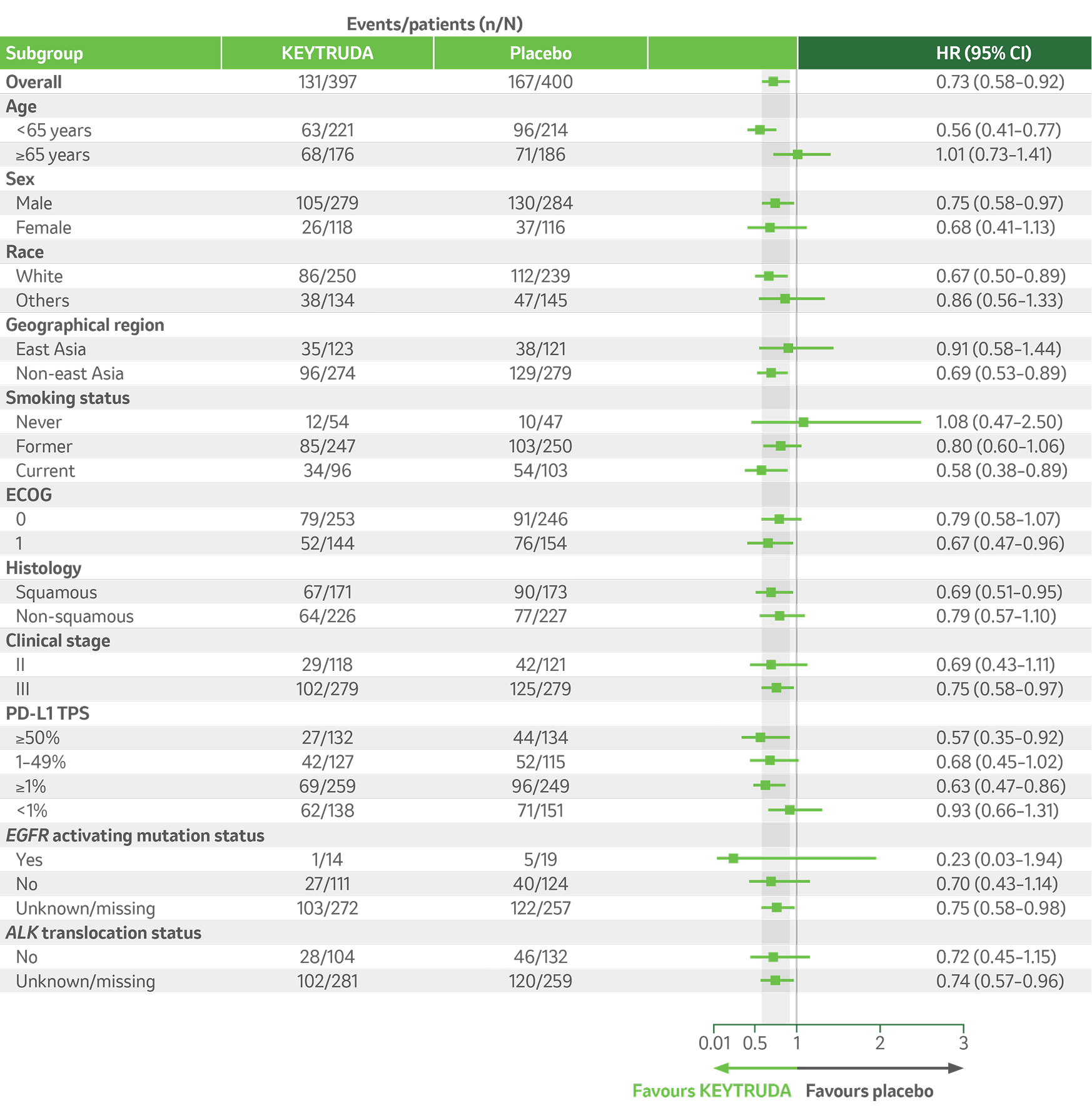
Adapted from Majem M, et al. 2024.5
Per the prespecified analysis plan, subgroups with <30 participants are excluded from the above forest plot. Subgroups for Stage IIIA and IIIB and pN status were post hoc; all other subgroups were prespecified.
IA3 dataset. Median follow-up was 41.1 months. Data cut-off date for IA3: 19 August 2024.5
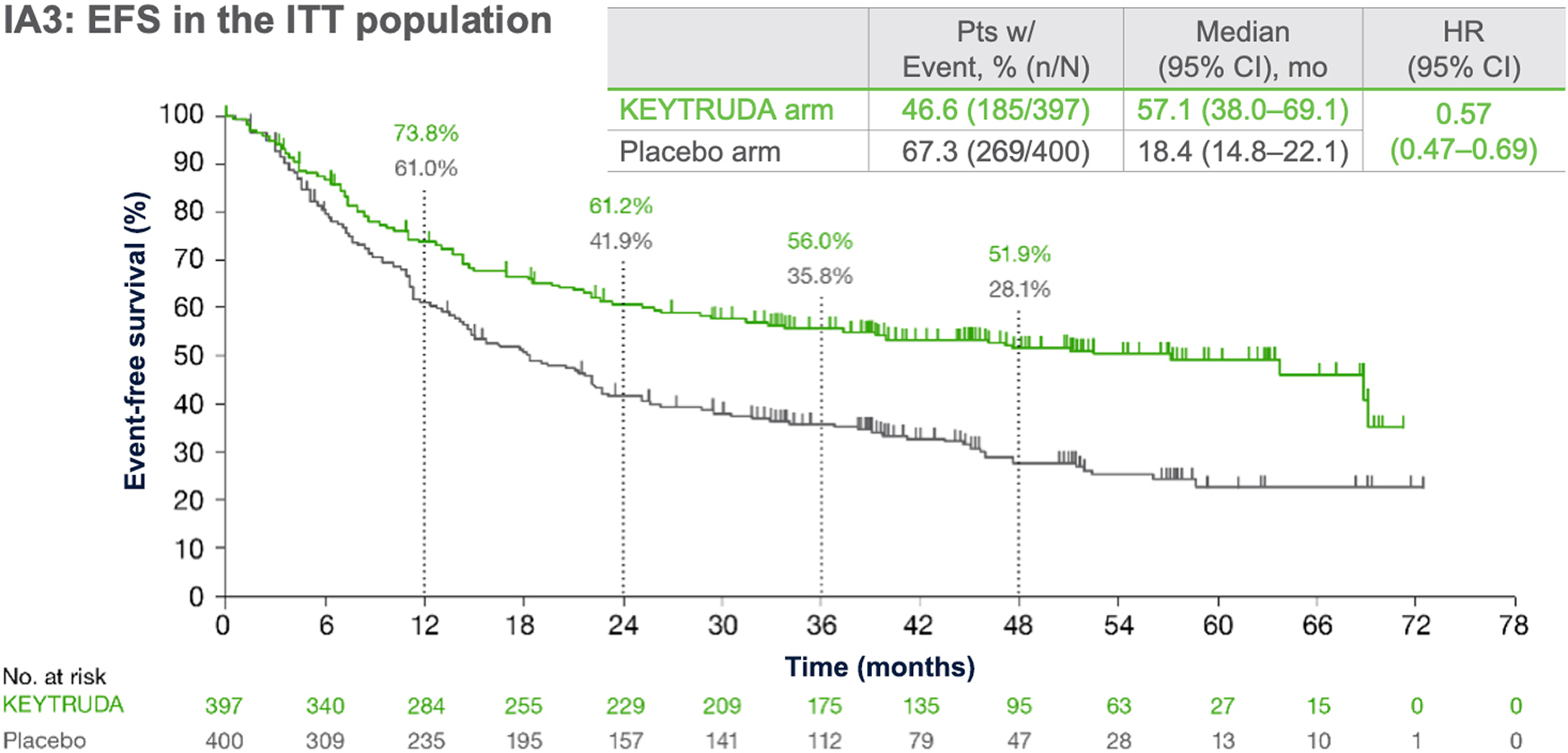
KEYNOTE-671: EFS in the ITT population (IA1, IA2 and IA3)*1,4,5
Dual primary endpoint
Median follow-up: 25.2 months (IA1); 36.6 months (IA2); 41.1 months (IA3)
Data cut-off date: 29 July 2022 (IA1); 10 July 2023 (IA2); 19 August 2024 (IA3)
-
At IA1, EFS benefit in the overall population was shown to be statistically significant and clinically meaningful1
- EFS HR was 0.58 (95% CI: 0.46–0.72); p<0.0011
- Median EFS was not reached in the perioperative KEYTRUDA arm and was 17 months in the placebo arm1
-
At IA2, with additional 11 months of follow-up, benefit was sustained with HR 0.594
- Median EFS was more than doubled, being 47.2 months with KEYTRUDA vs 18.3 months with placebo4
- 3-year EFS was 54% with pembrolizumab vs 35% with placebo4
Adapted from Majem M, et al. 2024.5
- At IA3, with an additional 16 months of follow-up, benefit was sustained with HR 0.575
Statistical testing for EFS was demonstrated at IA1, and therefore EFS was not formally tested at IA3.1,5
*Event-free survival defined as time from randomisation to first occurrence of local progression precluding planned surgery, unresectable tumour, progression or recurrence per RECIST version 1.1 by investigator assessment, or death from any cause.1
KEYNOTE-671 dual primary endpoint: EFS by subgroups in ITT populations5
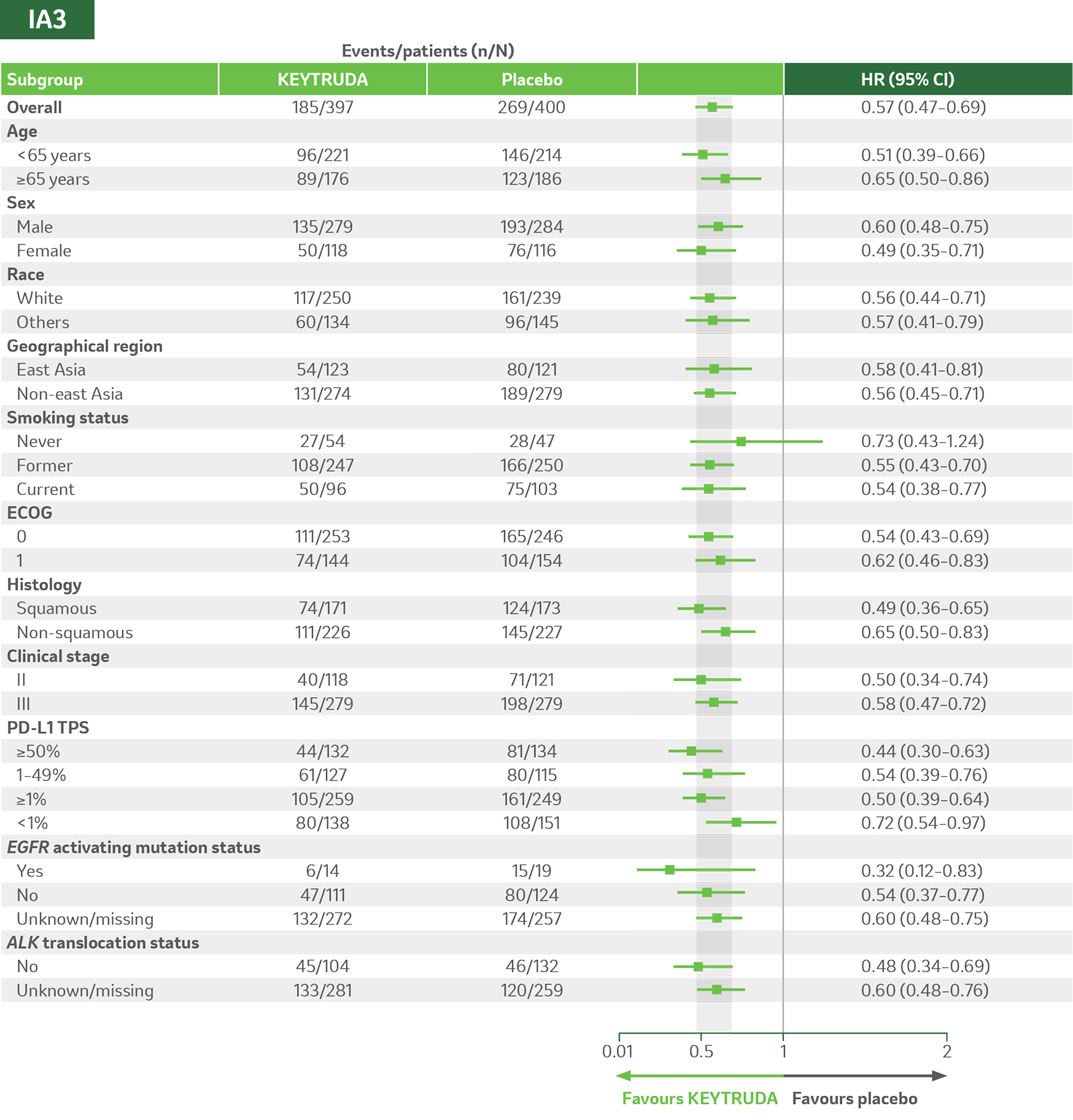
Adapted from Majem M, et al. 2024.5
Per the prespecified analysis plan, subgroups with <30 participants are excluded from the above forest plot. Subgroups for Stage IIIA and IIIB and pN status were post hoc; all other subgroups were prespecified.
Median follow-up was 41.1 months. Data cut-off for IA3: 19 August 2024.5
KEYNOTE-671 safety profile (as-treated population)
The safety profile of perioperative KEYTRUDA (plus chemotherapy) was consistent with the safety profiles of the individual components and no new safety signals were seen.1–5
IA3
Treatment-related adverse events (TRAEs)
Any grade5
96.7%
(n=383/396)
KEYTRUDA
vs
95.5%
(n=381/399)
Placebo
Grade ≥3*5
45.2%
(n=179/396)
KEYTRUDA
vs
37.8%
(n=151/399)
Placebo
Leading to treatment
discontinuation
19.4%
(n=77/396)
KEYTRUDA
vs
13.3%
(n=53/399)
Placebo
Leading to death
1.0%
(n=4/396)
KEYTRUDA
vs
0.8%
(n=3/399)
Placebo
IA3 dataset. Median follow-up was 41.1 months. Data cut-off date for IA3: 19 August 2024.5
*There were four deaths in the KEYTRUDA arm (atrial fibrillation, immune-mediated lung disease, pneumonia and sudden cardiac death). There were three deaths in the placebo arm (acute coronary syndrome, pneumonia and pulmonary haemorrhage). There have been no new treatment-related deaths during IA2 or IA3 since IA1.4,5
The most common TRAEs (incidence rate of ≥10%), across all treatment phases, in both KEYTRUDA and placebo groups (as-treated population)*
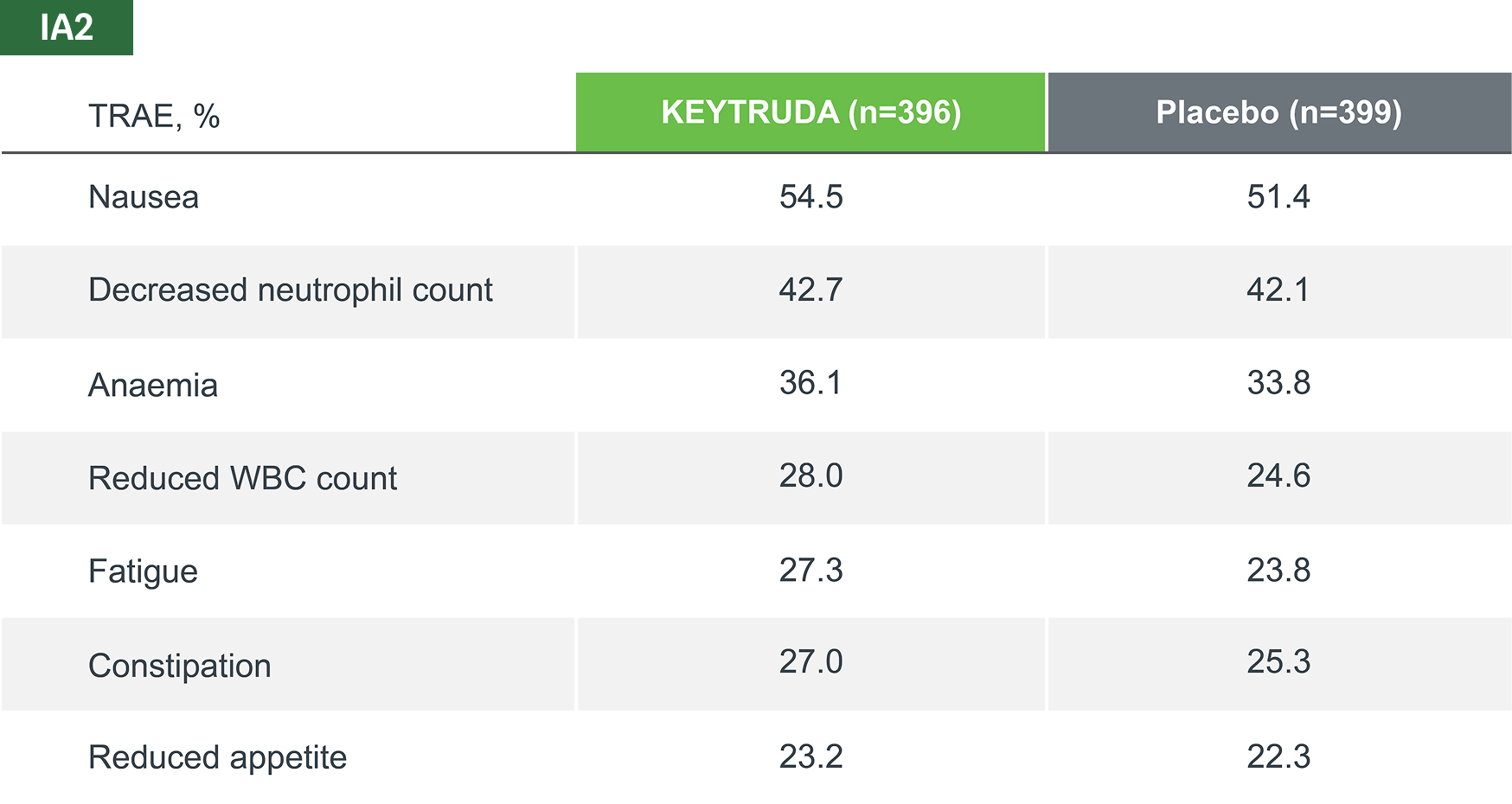
Adapted from Spicer JD, et al. 2023.4
TRAEs were AEs considered by the investigator to be related to chemotherapy, KEYTRUDA and placebo.
Median follow-up was 36.6 months. Data cut-off date for IA2: 10 July 2023.4
*IA2 data. Equivalent data was not presented at IA3.4
KEYNOTE-671 safety profile: Immune-mediated adverse events and infusion reactions
The incidence and nature of immune-mediated adverse events (IMAEs) in the KEYTRUDA arm were consistent with previous reports.5
IA3
IMAEs and infusion reactions
Any grade5
26.0%
(n=103/396)
KEYTRUDA
vs
9.3%
(n=37/399)
Placebo
Grade ≥3*5
6.3%
(n=25/396)
KEYTRUDA
vs
1.8%
(n=7/399)
Placebo
Leading to treatment
discontinuation
6.1%
(n=24/396)
KEYTRUDA
vs
0.8%
(n=3/399)
Placebo
Leading to death
0.3%
(n=1/396)
KEYTRUDA
vs
0%
(n=0/399)
Placebo
IA3 dataset. Median follow-up was 41.1 months. Data cut-off date for IA3: 19 August 2024.5
*There was one death in the KEYTRUDA arm (pneumonitis). There have been no new treatment-related deaths during IA2 or IA3 since IA1.4,5
The most common IMAEs and infusion reactions (occurring in ≥ 2 patients), across all treatment phases, in both KEYTRUDA and placebo groups (as-treated population)*4
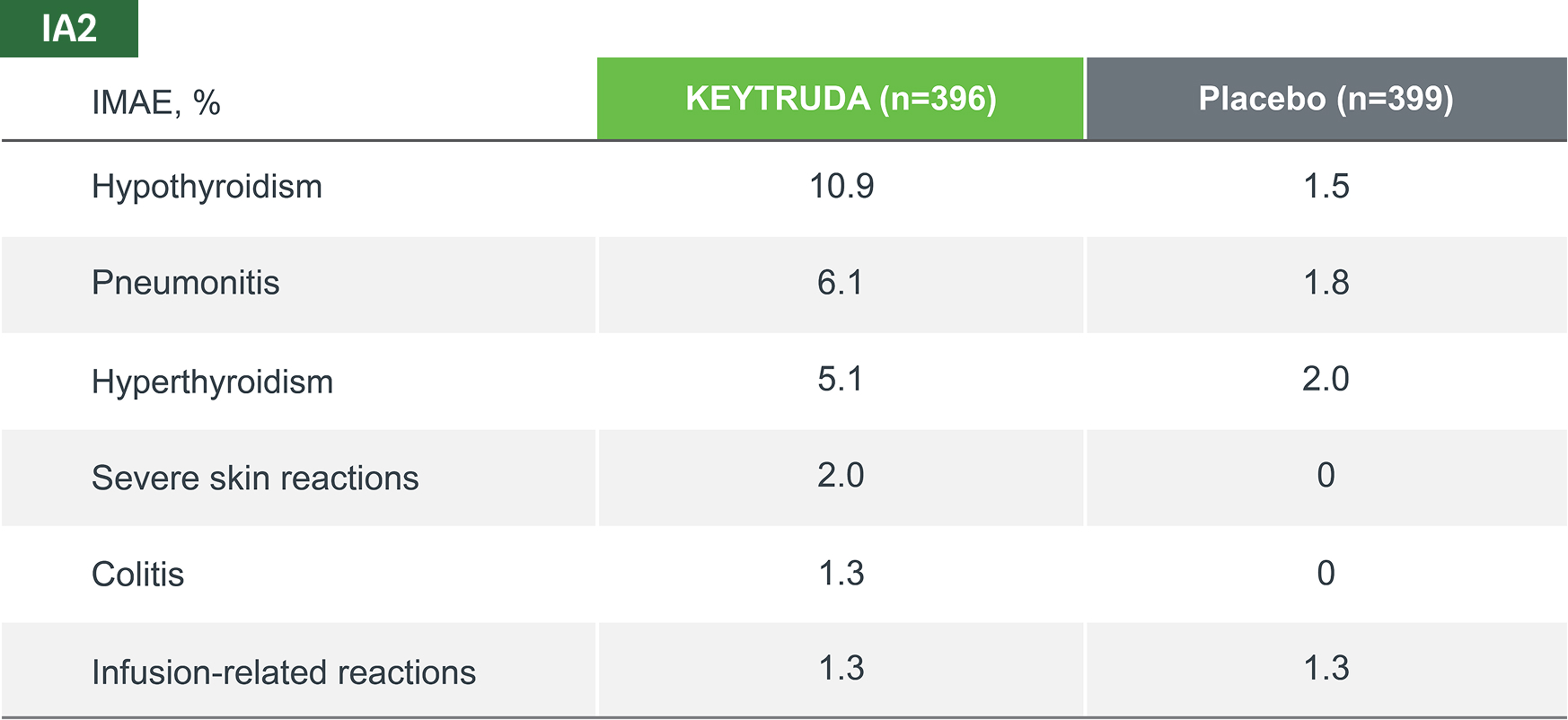
Adapted from Spicer JD, et al. 2023.4
IMAEs and infusion reactions were based on a list of preferred terms intended to capture known risks of KEYTRUDA and were considered regardless of attribution to study treatment by the investigator.
Median follow-up was 36.6 months. Data cut-off date for IA2: 10 July 2023.4
*IA2 data. Equivalent data was not presented at IA3.4
KEYTRUDA offers flexibility of dosing2



Assessment of regimens
The 200 mg Q3W (once every 3 weeks) regimen has been assessed in phase 2 and 3 registration studies across a multitude of indications of KEYTRUDA. An exposure-response evaluation, using modelling and simulation, led to the approval of the 400 mg Q6W (once every 6 weeks) dosing for monotherapy and combination therapy.2
From a microbiological point of view, the product, once diluted, should be used immediately. The diluted solution must not be frozen. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 7 days at 2˚C to 8˚C, or 12 hours at room temperature, unless dilution has taken place in controlled and validated aseptic conditions. If refrigerated, the vials and/or intravenous bags must be allowed to come to room temperature prior to use.2
For stability related enquiries please contact medicalinformationuk@msd.com
Explore our related resources
Sign up to MSD emails
✔ Get the latest product updates
✔ Receive cancer resources
✔ Be the first to hear about our events
KEYTRUDA early-stage and advanced NSCLC indications:2
- KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with NSCLC who are at high risk of recurrence following complete resection and platinum-based chemotherapy
- KEYTRUDA, in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment, is indicated for the treatment of resectable NSCLC at high risk of recurrence in adults
- KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic NSCLC in adults whose tumours express PD-L1 with a ≥50% TPS with no EGFR– or ALK-positive tumour mutations
- KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous NSCLC in adults whose tumours have no EGFR– or ALK-positive mutations
- KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous NSCLC in adults
- KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic NSCLC in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR– or ALK-positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA
Abbreviations
AE, adverse event; AJCC, American Joint Committee on Cancer; ALK, anaplastic lymphoma kinase; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EFS, event-free survival; EGFR, epidermal growth factor receptor; HR, hazard ratio; IA, interim analysis; IHC, immunohistochemistry; ILD, interstitial lung disease; IMAE, immune-mediated adverse event; ITT, intention-to-treat; IV, intravenous; mo, months; mPR, major pathological response; N2, involvement of the ipsilateral mediastinal and/or subcarinal lymph nodes;1 NR, not reached; NSCLC, non-small cell lung cancer; OS, overall survival; pCR, pathological complete response; PD-L1, programmed death-ligand 1; pN, pathological node involvement; Pt-CT, platinum-containing chemotherapy; Pts, patients; Q3W, every 3 weeks; Q6W, every 6 weeks; RECIST, Response Evaluation Criteria in Solid Tumours; TPS, tumour proportion score; TRAE, treatment-related adverse event; WBC, white blood cell.
References
- Wakelee H, et al. N Engl J Med 2023;389:491–503 (including protocol and supplementary materials).
- KEYTRUDA Summary of Product Characteristics. MSD. Available at: https://www.medicines.org.uk/emc/product/2498. Accessed: August 2025.
- Wakelee H, et al. KEYNOTE-671: Randomised, double-blind, Phase 3 study of pembrolizumab or placebo plus platinum-based chemotherapy followed by resection and pembrolizumab or placebo for early-stage NSCLC. ASCO. 2–6 June 2023. Chicago, Illinois, USA. Abstract: LBA100.
- Spicer JB, et al. Overall survival in the KEYNOTE-671 study of perioperative pembrolizumab for early-stage NSCLC. ESMO. 20–24 October 2023. Madrid, Spain. Abstract: LBA56.
- Majem M, et al. ESMO I-O 2024. 11–13 December 2024. Geneva, Switzerland. Abstract: LBA3.
Supporting documentation
Prescribing Information [External link]
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website.


