Adjuvant KEYTRUDA monotherapy
KEYTRUDA® (pembrolizumab) as adjuvant monotherapy following surgical resection and chemotherapy in early-stage non-small cell lung cancer1
Prescribing Information [External link]
The efficacy and safety of KEYTRUDA were investigated as adjuvant therapy for NSCLC in…
KEYNOTE-091
…a Phase III multicentre, randomised, triple-blind, placebo-controlled clinical study in 1177 patients with completely resected Stage IB* (T2a ≥4 cm), II or IIIA NSCLC1
During this trial, KEYTRUDA achieved the dual primary endpoint of DFS in all patients; the dual primary endpoint of DFS in PD-L1 TPS ≥50% patients was not mature but showed a favourable trend – see below for more details.1
*Tumour staging system as per AJCC 7th edition.1
Licensed indication
KEYTRUDA is licensed for use as a monotherapy for the adjuvant treatment of adults with NSCLC who are at high risk of recurrence following complete resection and platinum-based chemotherapy.2 The results of the ITT population are presented as this is the primary endpoint of the study. It allows the results in the licensed population (a subgroup of the ITT population) to be viewed in context of the primary endpoint.2
Key eligibility criteria:
- High risk of recurrence*
- Completely resected Stage IB (T2a ≥4 cm), II or IIIA NSCLC, regardless of PD-L1 expression (AJCC 7th edition)
- May or may not have received adjuvant chemotherapy (≤4 cycles) – to be considered for Stage IB disease and strongly recommended for Stage II and Stage IIIA disease
Key exclusion criteria:
- Prior neoadjuvant radiotherapy and/or neoadjuvant chemotherapy
- Prior or planned adjuvant radiotherapy for current malignancy
- Active autoimmune disease requiring systemic therapy in the past two years
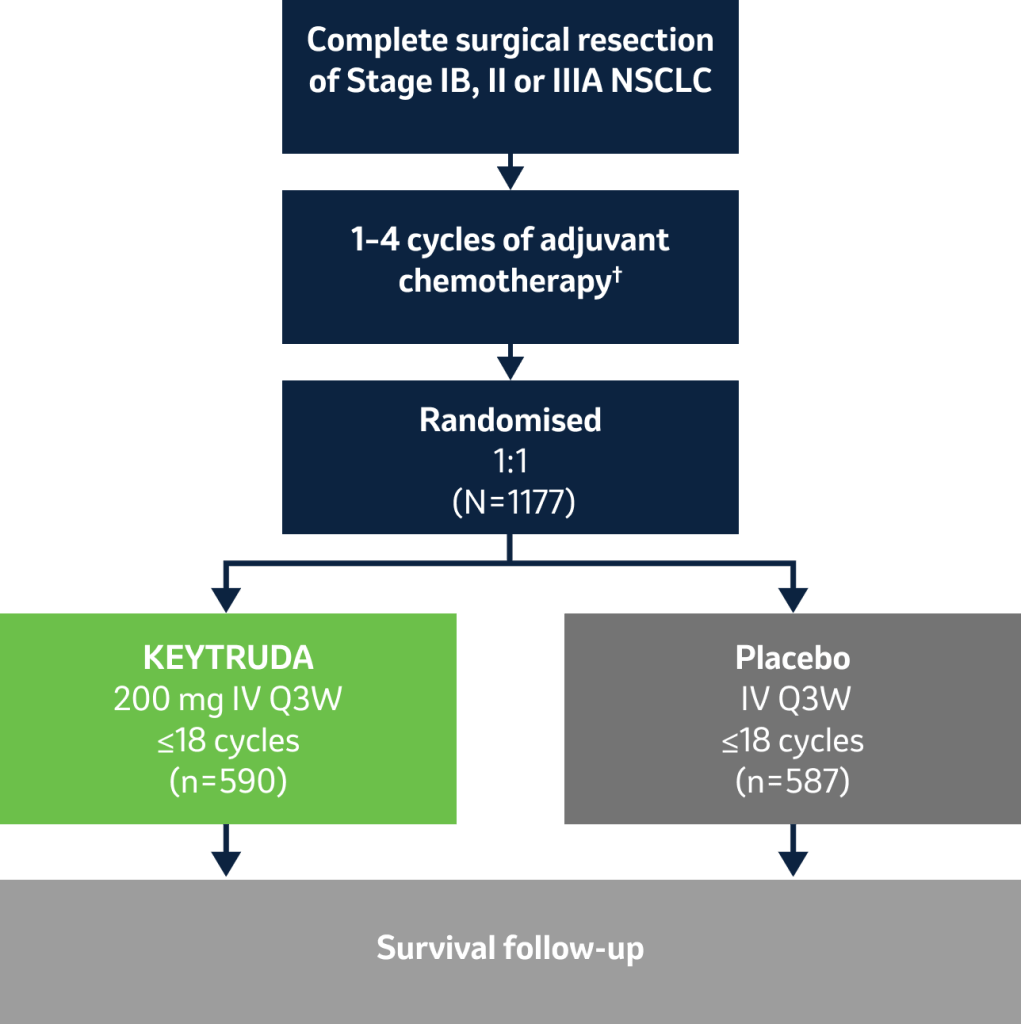
- Dual primary endpoints: DFS (all patients and PD-L1 TPS ≥50%)‡
- Secondary endpoints: DFS (PD-L1 TPS ≥1%), OS (all patients, PD-L1 TPS ≥50% and PD-L1 TPS ≥1%),‡ lung-cancer-specific survival (all patients), safety§
Stratification factors:
- Stage (IB vs II vs IIIA)
- Use of adjuvant chemotherapy (No vs Yes)
- PD-L1 status (TPS <1% vs 1 to 49% vs ≥50%)¶
- Regions (Western Europe vs Eastern Europe vs Asia vs rest of world)
*The following selection criteria define patients with high risk of recurrence who are included in the therapeutic indication and are reflective of the patient population with Stage IB (T2a ≥4 cm), II or IIIA according to the AJCC staging system (7th edition): tumour size ≥4 cm; or tumours of any size that are either accompanied by N1 or N2 status; or tumours that are invasive of thoracic structures (directly invade the parietal pleura, chest wall, diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium, mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, oesophagus, vertebral body and carina); or tumours that involve the main bronchus <2 cm distal to the carina but without involvement of the carina; or tumours that are associated with atelectasis or obstructive pneumonitis of the entire lung; or tumours with separate nodule(s) in the same lobe or different ipsilateral lobe as the primary. The study did not include patients who had N2 status with tumours also invading the mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, oesophagus, vertebral body and carina, or with separate tumour nodule(s) in a different ipsilateral lobe.2
†
Adjuvant chemotherapy was considered for Stage IB (T2a ≥4 cm in diameter) disease and strongly recommended for Stage II and IIIA disease, limited to ≤4 cycles.1
‡Disease-free survival was defined as time from randomisation to locoregional or metastatic recurrence assessed per RECIST version 1.1 by investigator review, appearance of a second NSCLC primary or other malignancy, or death from any cause, whichever occurred first.1
§OS was defined as time from randomisation to death from any cause.1
¶PD-L1 TPS status was assessed at a central laboratory using PD-L1 IHC 22C3 pharmDx.1
At IA2, the primary endpoint of DFS in all patients was met: KEYTRUDA monotherapy provided a significant DFS benefit vs placebo as adjuvant treatment in patients who are at high risk of recurrence following surgical resection and chemotherapy in early-stage NSCLC3
KEYNOTE-091: DFS in the ITT populations (IA2 and IA3)*3,4
Dual primary endpoint
Median follow-up: 32.4 months (IA2); 46.7 months (IA3)2,4
Data cut-off date: 20 September 2021 (IA2); 24 January 2023 (IA3)4
-
At IA2, adjuvant KEYTRUDA following complete resection and adjuvant chemotherapy (where recommended) significantly extended DFS in the overall population compared with placebo, with a 24% reduced risk of recurrence or death4
- Median DFS was 53.6 months with KEYTRUDA vs 42.0 months with placebo. HR was 0.76 (95% CI: 0.63–0.91), p=0.00143†‡
IA3: DFS in the overall ITT population3
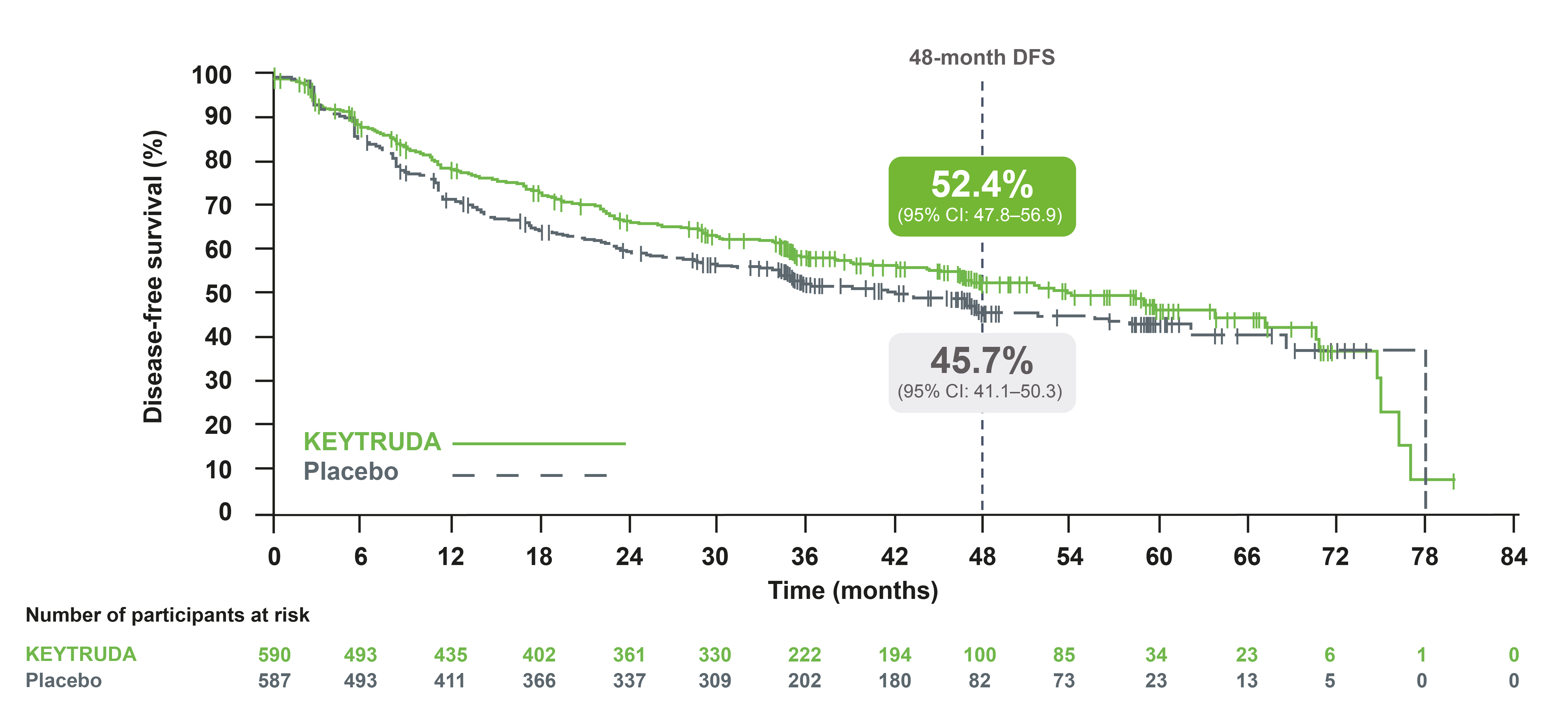
Adapted from Besse B, et al. 2023.3
-
At IA3, adjuvant KEYTRUDA continued to provide improved DFS in the overall population compared with placebo, with a 19% reduced risk of recurrence or death3
- Median DFS was 53.8 months with KEYTRUDA vs 43.0 months with placebo. HR was 0.81 (95% CI: 0.68–0.96)†‡
-
At IA2, DFS in the PD-L1 TPS ≥50% population was not mature but showed a favourable trend4
- Median DFS was not reached for both KEYTRUDA and placebo. HR was 0.82 (95% CI: 0.57–1.18), p=0.13639†‡
IA3: DFS in the PD-L1 TPS ≥50% population3
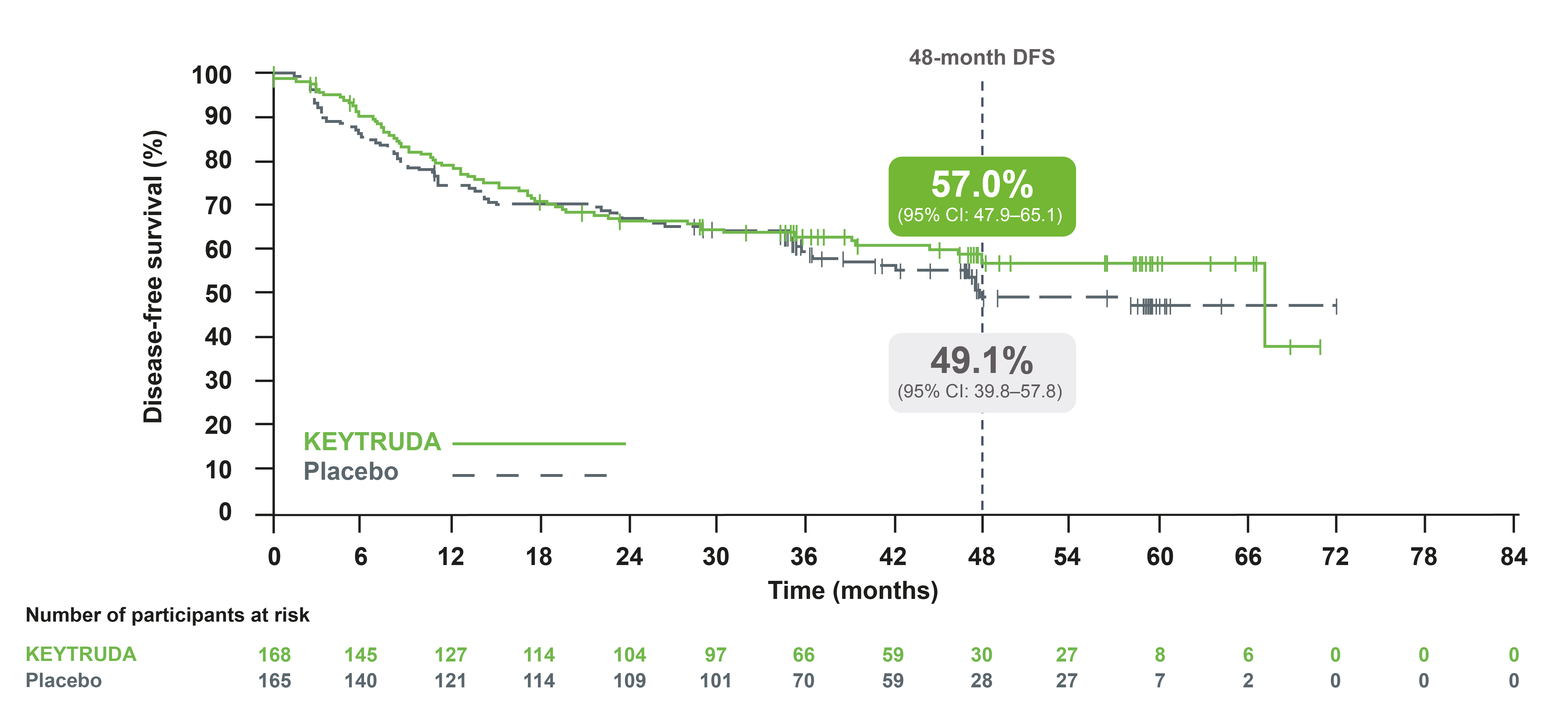
Adapted from Besse B, et al. 2023.3
-
At IA3, DFS in the PD-L1 TPS ≥50% population was still not mature but continued to show a favourable trend3
- Median DFS was 67.0 months with KEYTRUDA vs 47.6 months with placebo. HR was 0.83 (95% CI: 0.59–1.16), p=0.13‡
*Investigator-assessed DFS was defined as the time between the date of randomisation and the date of first recurrence (local regional recurrence, distant metastasis), a second malignancy or death, whichever occurred first.1
†Based on the multivariate Cox regression model with treatment adjusted by the following covariates: Stage (IB vs II vs IIIA), PD-L1 status (≥50% vs 1–49% vs <1%), region (Western Europe vs Eastern Europe vs rest of world vs Asia), histology (squamous vs non-squamous), and smoking status (never vs former/current).4
‡One-sided p-values for DFS endpoints are based on the permutation test with the multivariate Cox regression model; one-sided p-values for OS endpoints are based on the Wald test in the multivariate Cox regression model.4
KEYTRUDA extends DFS vs placebo in patients who are at high risk of recurrence following complete resection and platinum-based chemotherapy1,3
KEYNOTE-091 exploratory analysis: DFS in patients who received adjuvant chemotherapy – UK licensed indication (IA2 and IA3)*3
Exploratory analysis
Median follow-up: 32.4 months (IA2); 46.7 months (IA3)2,4
Data cut-off date: 20 September 2021 (IA2); 24 January 2023 (IA3)4
-
At IA2, adjuvant KEYTRUDA following complete resection and adjuvant chemotherapy (where recommended) extended DFS in patients who received prior adjuvant chemotherapy, compared with placebo1
- DFS HR was 0.73 (95% CI: 0.60–0.89)†
IA3: DFS in the prior adjuvant chemotherapy population3
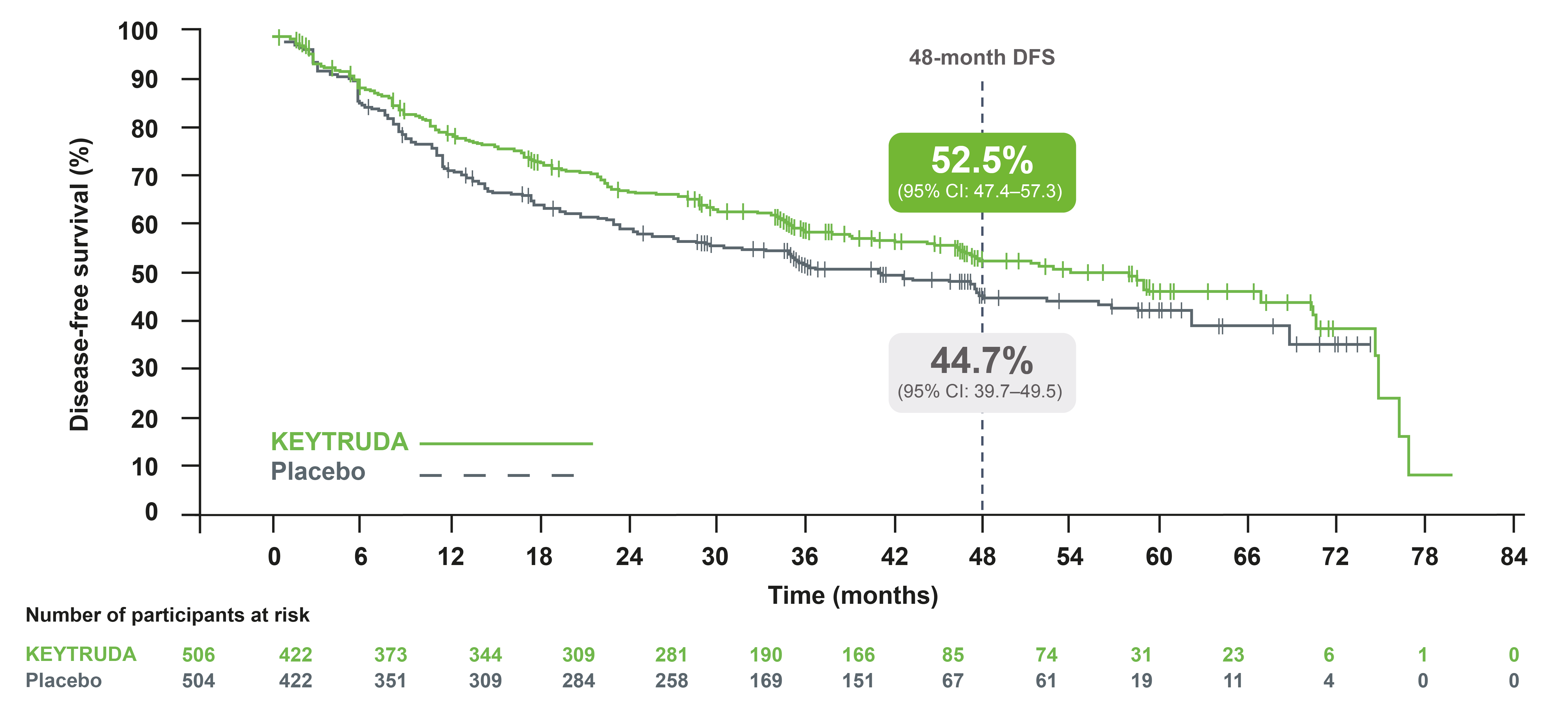
Adapted from Besse B, et al. 2023.3
-
At IA3, adjuvant KEYTRUDA continued to provide improved DFS in the patients who received adjuvant chemotherapy compared with placebo, with a 24% reduced risk of recurrence or death4
- Median DFS was 53.8 months with KEYTRUDA vs 40.5 months with placebo. HR was 0.76 (95% CI: 0.64–0.91, one sided p=0.00150)†
*Investigator-assessed DFS was defined as the time between the date of randomisation and the date of first recurrence (local regional recurrence, distant metastasis), a second malignancy or death, whichever occurred first.1
†Based on Cox model with treatment as covariate.1,4
KEYNOTE-091 exploratory analysis: DFS by key subgroups in patients who received adjuvant chemotherapy (IA3 analysis)4
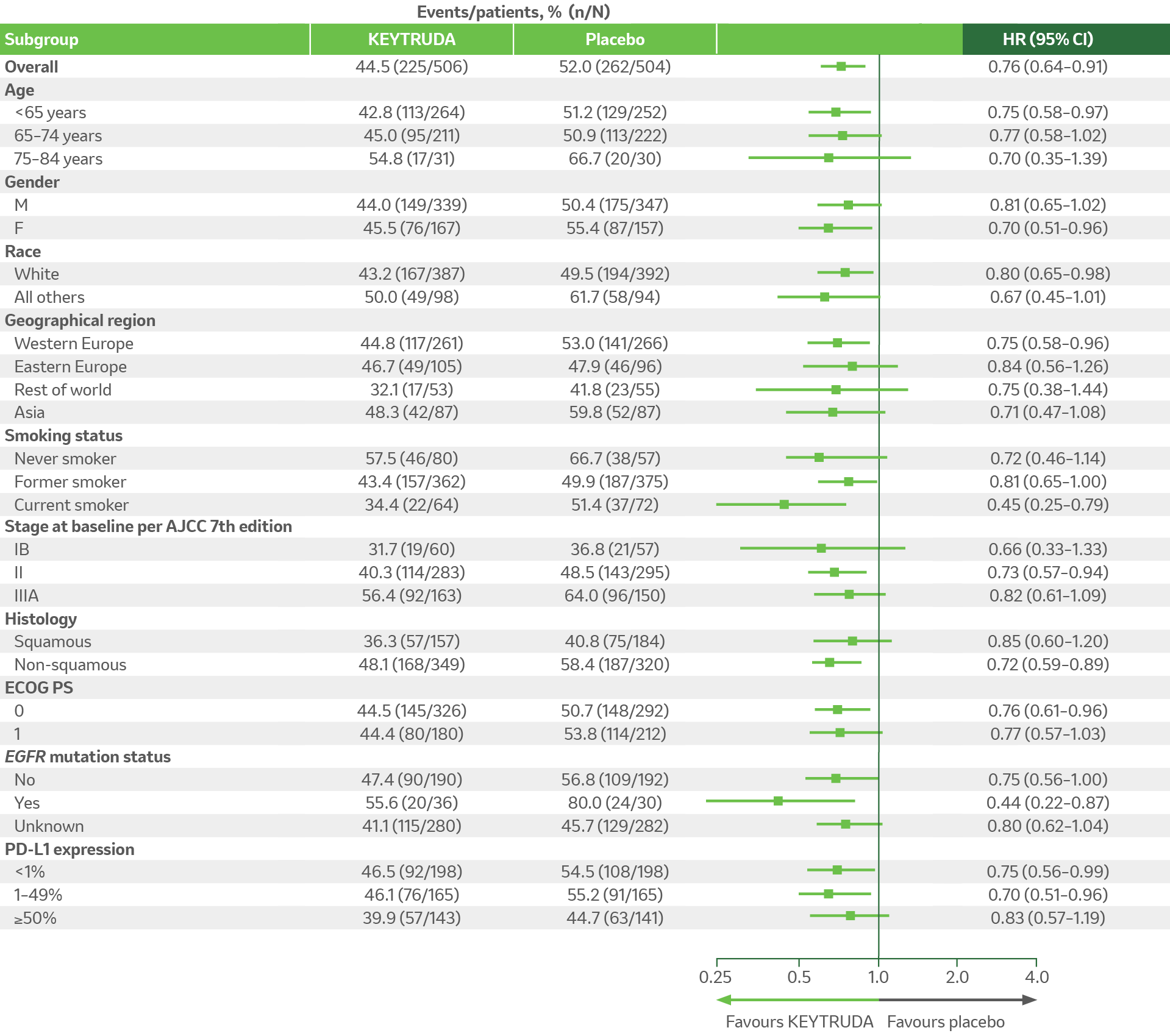
Adapted from KN-091 EPAR report 2023.4
For overall population and all subgroups, analysis is based on multivariate Cox regression model with treatment adjusted by the following covariates: Stage (IB vs II vs IIIA), PD-L1 status (≥50% vs 1–49% vs <1%), region (Western Europe vs Eastern Europe vs rest of world vs Asia), histology (squamous vs non-squamous), and smoking status (never vs former/current). If the number of participants in a category of a subgroup variable is less than 50 (except EGFR mutation status), or the number of events in a category of a subgroup variable is zero in one treatment arm, or the number of events in a category of a subgroup variable is less than 5 in the pooled arms, the subgroup analysis will not be performed for this category of the subgroup variable.4
KEYNOTE-091 safety profile (as-treated population)
Summary of AEs in all treated patients (IA3 analysis)3
Median follow-up: 46.7 months4
Data cut-off date: 24 January 20234
The AE profile observed with KEYTRUDA was similar to that in previous studies of KEYTRUDA monotherapy, including of locally advanced or metastatic NSCLC and of adjuvant therapy for melanoma and renal cell carcinoma.1,3
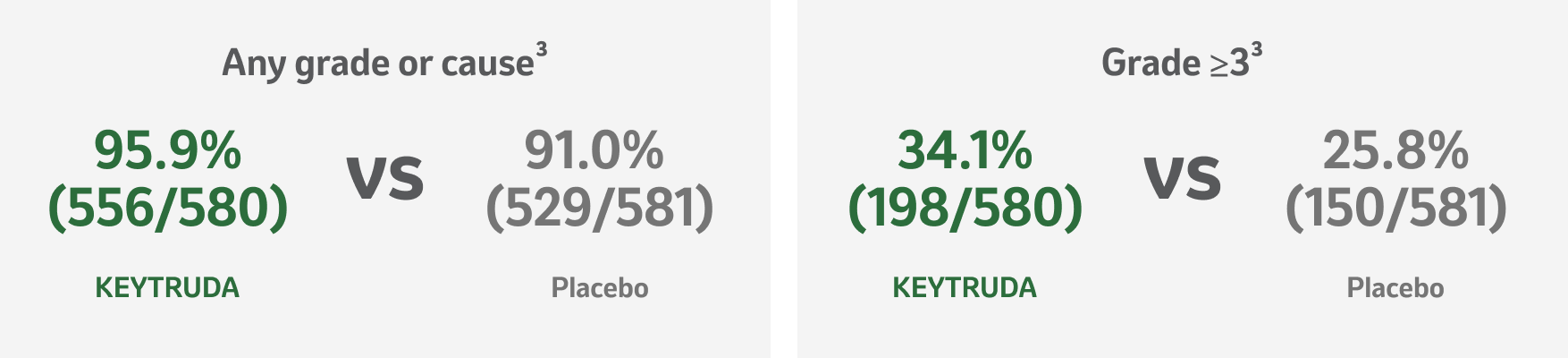
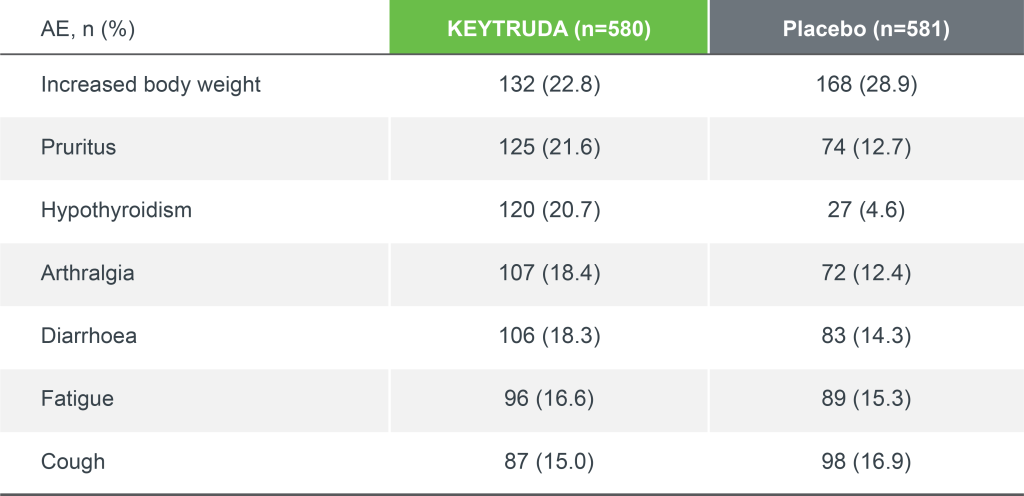
Adapted from Besse B, et al. 2023.3
*At time of final DFS analysis; IA3 data set.3
KEYNOTE-091 safety profile: Immune-mediated adverse events and infusion reactions
Median follow-up: 46.7 months4
Data cut-off date: 24 January 20234
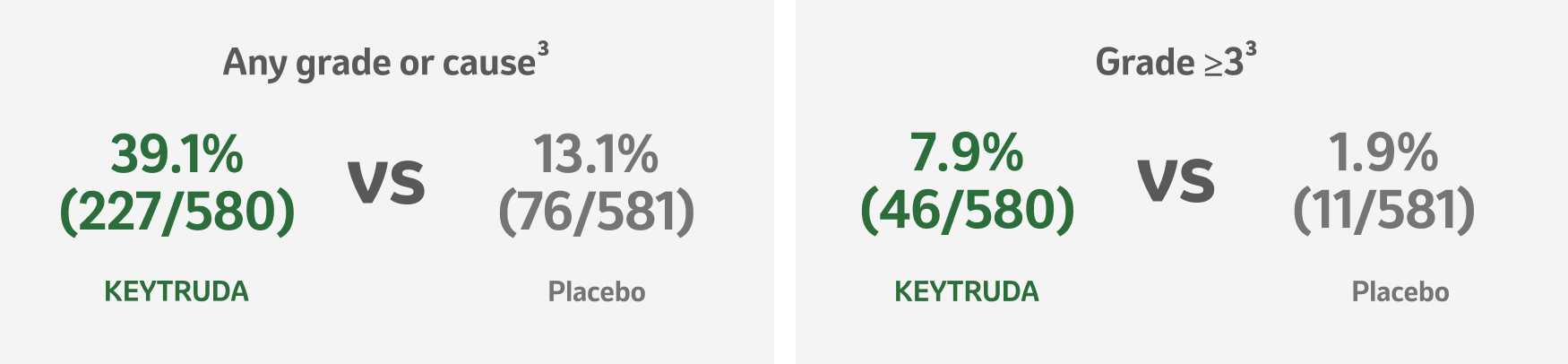
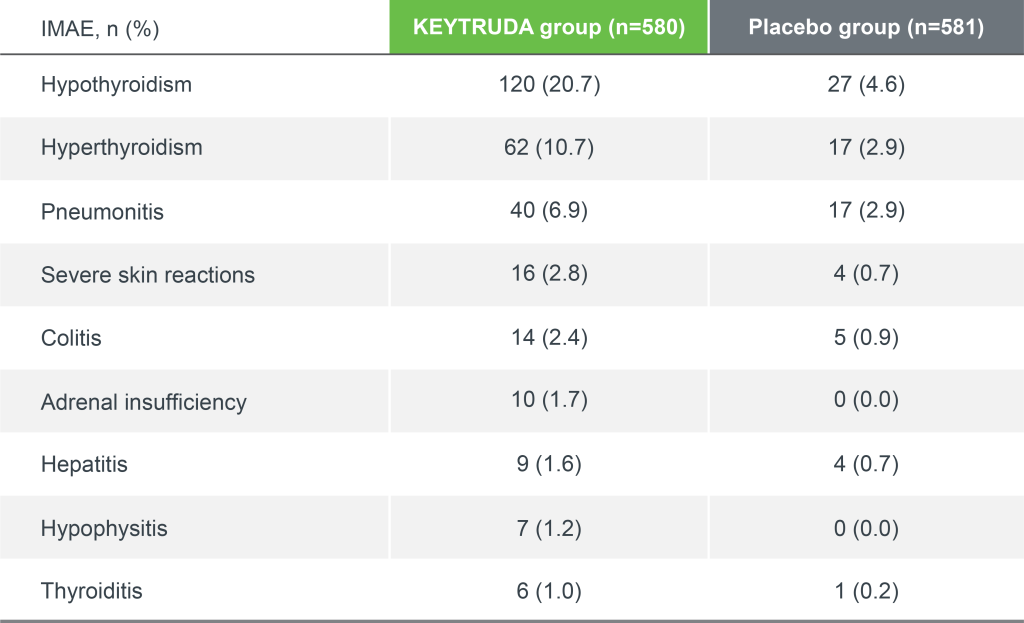
Adapted from Besse B, et al. 2023.3
*IMAEs and infusion reactions were based on a list of preferred terms intended to capture known risks of KEYTRUDA and were considered regardless of attribution to study treatment by the investigator.3
KEYTRUDA offers flexibility of dosing2



Assessment of regimens
The 200 mg Q3W (once every 3 weeks) regimen has been assessed in Phase 2 and 3 registration studies across a multitude of indications of KEYTRUDA. An exposure-response evaluation, using modelling and simulation, led to the approval of the 400 mg Q6W (once every 6 weeks) dosing for monotherapy and combination therapy.2
From a microbiological point of view, the product, once diluted, should be used immediately. The diluted solution must not be frozen. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 7 days at 2˚C to 8˚C, or 12 hours at room temperature, unless dilution has taken place in controlled and validated aseptic conditions. If refrigerated, the vials and/or intravenous bags must be allowed to come to room temperature prior to use.2
For stability related enquiries please contact medicalinformationuk@msd.com
Explore our related resources
Sign up to MSD emails
✔ Get the latest product updates
✔ Receive cancer resources
✔ Be the first to hear about our events
KEYTRUDA early-stage and advanced NSCLC indications:2
- KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with NSCLC who are at high risk of recurrence following complete resection and platinum-based chemotherapy
- KEYTRUDA, in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment, is indicated for the treatment of resectable NSCLC at high risk of recurrence in adults
- KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic NSCLC in adults whose tumours express PD-L1 with a ≥50% TPS with no EGFR– or ALK-positive tumour mutations
- KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous NSCLC in adults whose tumours have no EGFR- or ALK-positive mutations
- KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous NSCLC in adults
- KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic NSCLC in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR- or ALK-positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA
- Please consult the Summary of Product Characteristics for further information to minimise the risks associated with the use of KEYTRUDA before making prescribing decisions.
Abbreviations
AE, adverse event; AJCC, American Joint Committee on Cancer; ALK, anaplastic lymphoma kinase; CI, confidence interval; DFS, disease-free survival; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; HR, hazard ratio; IA, interim analysis; IHC, immunohistochemistry; IMAE, immune-mediated adverse event; ITT, intention-to-treat; IV, intravenous; NSCLC, non-small-cell lung carcinoma; OS, overall survival; PD-L1, programmed death-ligand 1; Q3W, every 3 weeks; Q6W, every 6 weeks; RECIST, Response Evaluation Criteria In Solid Tumours; SmPC, Summary of Product Characteristics; TPS, tumour proportion score.
References
- O’Brien M, et al. Lancet Oncol 2022;23:1274–1286.
- KEYTRUDA Summary of Product Characteristics. MSD. Available at: https://www.medicines.org.uk/emc/product/2498/smpc. Accessed: October 2025.
- Besse B, et al. Adjuvant pembrolizumab versus placebo for early-stage NSCLC after resection and optional chemotherapy: Updated results from PEARLS/KEYNOTE-091. ESMO. 6–8 December 2023. Geneva, Switzerland.
- European Medicines Agency. European public assessment report: KEYTRUDA. Available at: https://www.ema.europa.eu/en/documents/variation-report/keytruda-h-c-003820-ii-0121-epar-assessment-report-variation_en.pdf. Accessed: October 2025.
Supporting documentation
Prescribing Information (United Kingdom) [External link]
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website.


