KEYTRUDA as monotherapy in previously treated, PD-L1 positive mNSCLC
KEYTRUDA® (pembrolizumab) as monotherapy in previously treated, PD-L1 positive, metastatic non-small cell lung cancer1,2
Prescribing Information [External link]
The efficacy and safety of KEYTRUDA monotherapy was assessed in previously treated, PD-L1 positive, metastatic NSCLC in…
KEYNOTE-010
…a randomised, controlled, open-label Phase II/III clinical trial in 1034 patients with previously treated, PD-L1 positive metastatic NSCLC1,2
During this trial, KEYTRUDA achieved the primary endpoint of OS and demonstrated numerically longer PFS – see below for more details.1,2
Licensed indication
KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic non-small cell lung carcinoma in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR– or ALK-positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA.3
Key eligibility criteria:
- Histologically or cytologically confirmed Stage IV NSCLC
- Measurable disease and progression per RECIST v1.1 after chemotherapy
- ECOG PS 0/1
- PD-L1 TPS ≥1%
Key exclusion criteria:
- No active brain metastasis, carcinomatous meningitis, autoimmune disease, interstitial lung disease or history of pneumonitis
- Previous treatment with PD-1 checkpoint inhibitors or docetaxel
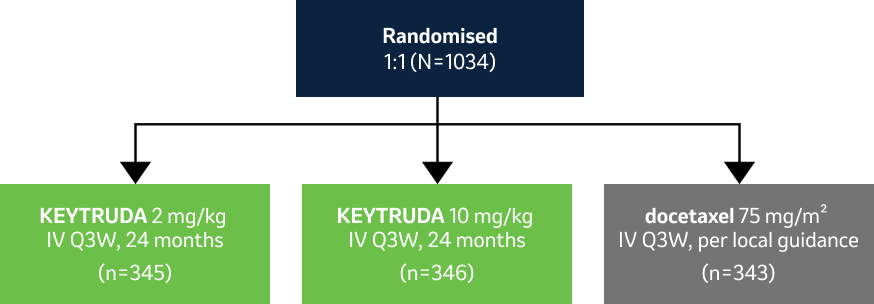
Please note this study design used weight-based dosing. This is no longer within the license due to a switch to fixed dosing.3
Please refer to the SmPC for current dosing.
- Primary endpoints: OS, PFS in the ITT and ≥50% TPS populations
- Secondary endpoints: Safety, response rate, DOR
Stratification factors:
- ECOG PS 0 vs 1
- PD-L1 TPS ≥50% vs 1–49%
- East Asian vs non-East Asian site
In the primary analysis (IA1) at a median follow-up of 13.1 months, KEYTRUDA monotherapy demonstrated survival benefit1
KEYNOTE-010: OS in the PD-L1 TPS ≥1% population (IA1 and 5-year analysis)1,2
Dual primary endpoint
Median follow-up: 13.1 months (IA1); 67.4 months (5-year analysis).1,2
Data cut-off: 30 September 2015 (IA1); 8 April 2020 (5-year analysis).1,2
-
At IA1, KEYTRUDA demonstrated a superior OS benefit vs docetaxel:1
- KEYTRUDA 2 mg/kg demonstrated a 29% reduced risk of death vs docetaxel (HR: 0.71; 95% CI: 0.58–0.88), p=0.0008
- Median OS was 10.4 months (95% CI: 9.4–11.9) with KEYTRUDA 2 mg/kg vs 8.5 months (95% CI: 7.5–9.8) with docetaxel
- KEYTRUDA 10 mg/kg demonstrated a 39% reduced risk of death vs docetaxel (HR: 0.61; 95% CI: 0.49–0.75), p<0.0001
- Median OS was 12.7 months (95% CI: 10.0–17.3) with KEYTRUDA 10 mg/kg vs 8.5 months (95% CI: 7.5–9.8) with docetaxel
-
At the 5-year analysis, OS benefit was sustained with KEYTRUDA (2 mg/kg and 10 mg/kg pooled) vs docetaxel2
- No statistical conclusions can be drawn from exploratory analyses
- KEYTRUDA demonstrated a 30% reduced risk of progression vs docetaxel (95% CI: 0.61–0.80)
- Median OS was 11.8 months (95% CI: 10.4–13.1) with KEYTRUDA vs 8.4 months (95% CI: 7.6–9.5) with docetaxel
KEYNOTE-010: PFS in the PD-L1 TPS ≥1% population (IA1 and 5-year analysis)1,2
Dual primary endpoint
Median follow-up: 13.1 months (IA1); 67.4 months (5-year analysis).1,2
Data cut-off: 30 September 2015 (IA1); 8 April 2020 (5-year analysis).1,2
-
At IA1, KEYTRUDA did not achieve statistical significance in the ITT population vs docetaxel:1
- KEYTRUDA 2 mg/kg demonstrated a 12% reduced risk of progression vs docetaxel (HR: 0.88; 95% CI: 0.74–1.05), p=0.07
- Median PFS was 3.9 months (95% CI: 3.1–4.1) with KEYTRUDA 2 mg/kg vs 4.0 months (95% CI: 3.1–4.2) with docetaxel
- KEYTRUDA 10 mg/kg demonstrated a 21% reduced risk of progression vs docetaxel (HR: 0.79; 95% CI: 0.66–0.94), p=0.004
- Median PFS was 4.0 months (95% CI: 2.7–4.3) with KEYTRUDA 10 mg/kg vs 4.0 months (95% CI: 3.1–4.2) with docetaxel
-
At the 5-year analysis, KEYTRUDA demonstrated a PFS benefit (2 mg/kg and 10 mg/kg pooled) vs docetaxel (not statistically significant)2
- No statistical conclusions can be drawn from exploratory analyses
- KEYTRUDA demonstrated a 16% reduced risk of progression vs docetaxel (95% CI: 0.73–0.96)
- Median PFS was 4.0 months (95% CI: 3.1–4.1) with KEYTRUDA vs 4.1 months (95% CI: 3.8–4.5) with docetaxel
KEYNOTE-010 safety profile (as-treated population)2
Summary of AEs in all treated patients (5-year update)2
Median follow-up: 67.4 months.2
Data cut-off: 8 April 2020.2
KEYTRUDA had a generally manageable safety profile with no new safety signals identified for KEYTRUDA with long-term follow-up.2
AEs reported in subjects who received KEYTRUDA, received docetaxel and completed 35 cycles or 2 years of KEYTRUDA (data cut-off date: 8 April 2020)2
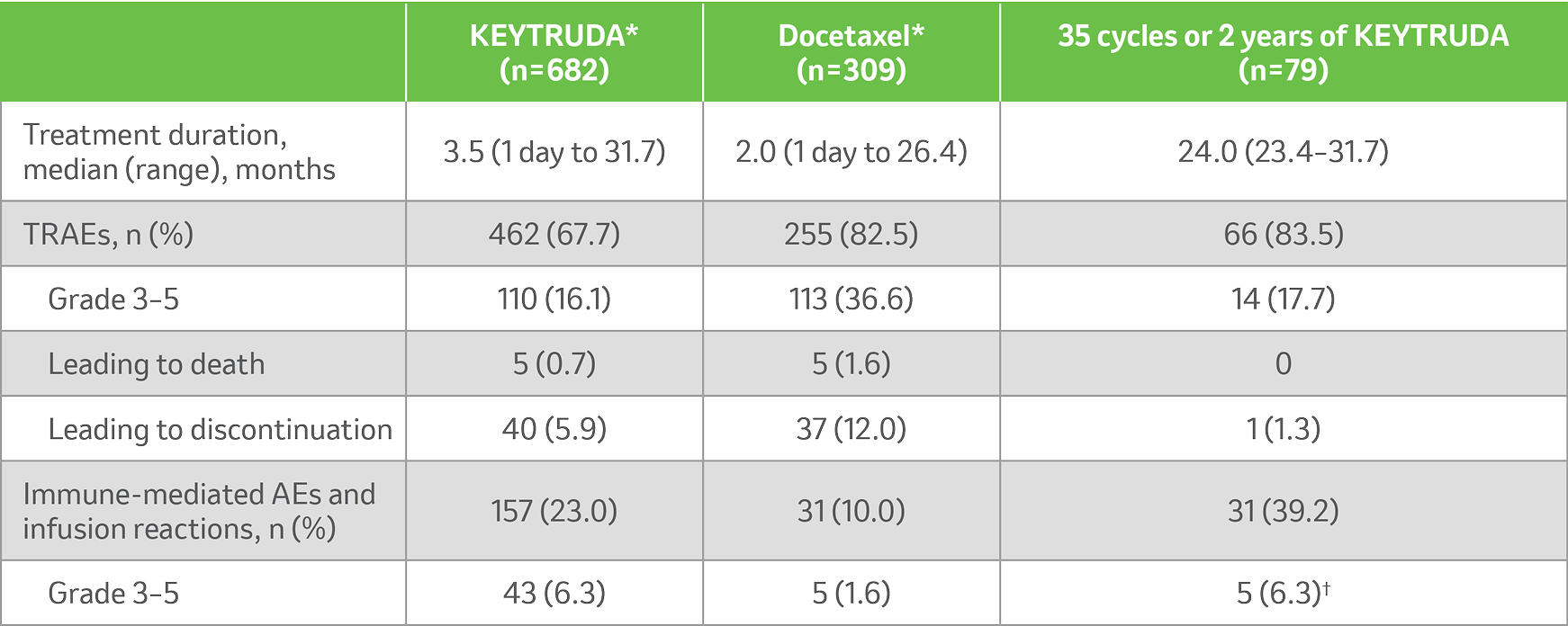
*During treatment with the initially assigned therapy.2
†IMAEs were Grade 3/4.2
KEYTRUDA offers flexibility of dosing3



Assessment of regimens
The 200 mg Q3W regimen has been assessed in Phase II and III registration studies across a multitude of indications of KEYTRUDA. An exposure-response evaluation, using modelling and simulation, led to the approval of the 400 mg Q6W dosing for monotherapy and combination therapy.3
From a microbiological point of view, the product, once diluted, should be used immediately. The diluted solution must not be frozen. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 7 days at 2˚C to 8˚C, or 12 hours at room temperature, unless dilution has taken place in controlled and validated aseptic conditions. If refrigerated, the vials and/or intravenous bags must be allowed to come to room temperature prior to use.3
For stability related enquiries please contact medicalinformationuk@msd.com
Explore our related resources
Sign up to MSD emails
✔ Get the latest product updates
✔ Receive cancer resources
✔ Be the first to hear about our events
KEYTRUDA early-stage and advanced NSCLC indications:3
- KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with NSCLC who are at high risk of recurrence following complete resection and platinum-based chemotherapy
- KEYTRUDA, in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment, is indicated for the treatment of resectable NSCLC at high risk of recurrence in adults
- KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic NSCLC in adults whose tumours express PD-L1 with a ≥50% TPS with no EGFR– or ALK-positive tumour mutations
- KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous NSCLC in adults whose tumours have no EGFR– or ALK-positive mutations
- KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous NSCLC in adults
- KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic NSCLC in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR– or ALK-positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA
-
Please consult the Summary of Product Characteristics for further information to minimise the risks associated with the use of KEYTRUDA before making prescribing decisions.
Abbreviations
AE, adverse event; ALK, anaplastic lymphoma kinase; CI, confidence interval; DOR, duration of response; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; HR, hazard ratio; IA, interim analysis; ITT, intention to treat; IV, intravenous; mNSCLC, metastatic non-small cell lung cancer; NSCLC, non-small cell lung cancer; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PFS, progression-free survival; Q3W, every 3 weeks; Q6W, every 6 weeks; RECIST, Response Evaluation Criteria in Solid Tumours; SmPC, Summary of Product Characteristics; TPS, tumour proportion score; TRAE, treatment-related adverse event.
References
- Herbst RS, et al. Lancet 2016;387:1540–1550.
- Herbst RS, et al. J Thorac Oncol 2021;16:1718–1732.
- KEYTRUDA Summary of Product characteristics. MSD. Available at: https://www.medicines.org.uk/emc/product/2498/smpc Accessed: September 2025.
- Herbst RS, et al. Lancet 2016;387:1540–1550. Supplementary appendix.
Supporting documentation
Prescribing Information [External link]
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website.

