KEYTRUDA monotherapy in PD-L1 ≥50% mNSCLC
KEYTRUDA® (pembrolizumab) as monotherapy for the first-line treatment of metastatic, PD-L1 ≥50% TPS, EGFR/ALK-wild-type non-small cell lung cancer1
Prescribing Information [External link]
The efficacy and safety of KEYTRUDA were investigated as first-line treatment in metastatic NSCLC in…
KEYNOTE-024
…a Phase III, randomised, open-label clinical study in 305 patients with untreated Stage IV NSCLC whose tumours express PD-L1 with a ≥50% TPS and no EGFR– or ALK-positive mutations.1
During this trial, KEYTRUDA achieved the primary endpoint of PFS – see below for more details.1
Licensed indication
KEYTRUDA as monotherapy is indicated for the first-line treatment of mNSCLC in adults whose tumours express PD-L1 with a ≥50% TPS with no EGFR- or ALK-positive tumour mutations.2
Key eligibility criteria:
- Untreated Stage IV NSCLC
- PD-L1 TPS ≥50%
- ECOG PS 0–1
Key exclusion criteria:
- Sensitising EGFR mutation or ALK translocation
- Untreated brain metastases
- Active autoimmune disease requiring systemic therapy
- Active immune ILD or pneumonitis requiring systemic therapy
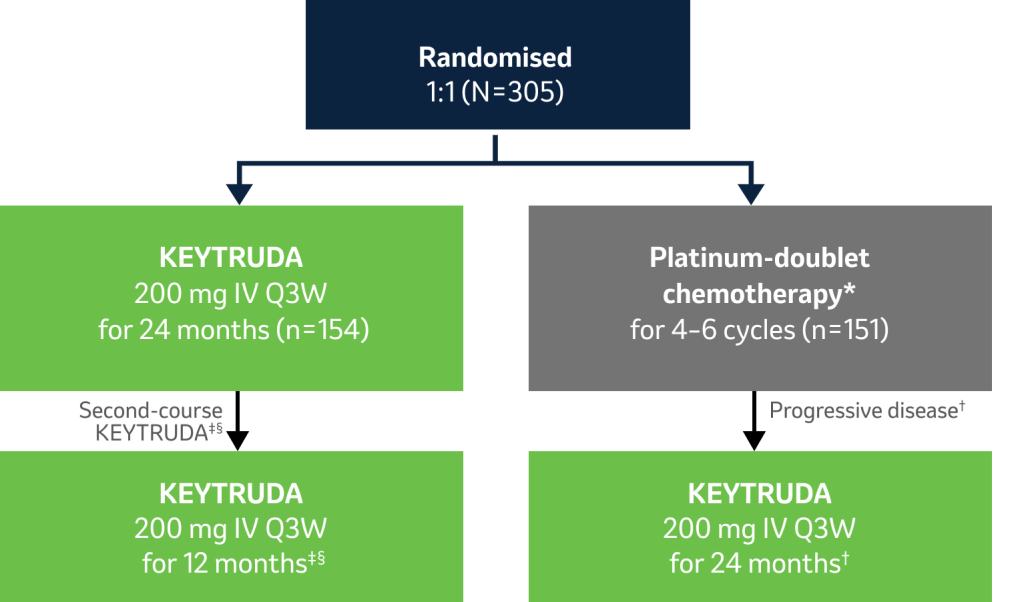
- Primary endpoint: PFS (RECIST v1.1 per blinded, independent central review)
- Secondary endpoints: OS, ORR, safety
- Exploratory: DOR
*Investigator’s choice of one of the following five platinum-based chemotherapy regimens for 4–6 cycles: carboplatin plus pemetrexed, cisplatin plus pemetrexed, carboplatin plus gemcitabine, cisplatin plus gemcitabine, or carboplatin plus paclitaxel.1
†To be eligible for crossover, PD had to be confirmed by blinded, independent central radiology review and all safety criteria had to be met.1
‡Patients were eligible for a second course of KEYTRUDA (up to 17 cycles) if PD occurred after completion of 35 cycles of KEYTRUDA or after attaining confirmed CR with at least 6 months of treatment and an additional two cycles of KEYTRUDA after CR was achieved and anticancer therapy had not been administered since their last dose of KEYTRUDA and protocol-specified eligibility criteria were met.3
§KEYTRUDA is not reimbursed after two years of treatment as recommended by NICE and the SMC.4,5
At IA1, the primary endpoint of PFS was met: KEYTRUDA provided a significant survival benefit vs platinum-based chemotherapy for the first-line treatment of metastatic, PD-L1 ≥50% TPS, EGFR/ALK-wild-type NSCLC in adults1
KEYNOTE-024: PFS in the ITT population (IA1 and 5-year analysis)*†1,3
Primary endpoint
Median follow up: 11.2 months (IA1); 59.9 months (5-year analysis)1,3
Data cut-off date: 9 May 2016 (IA1); 1 June 2020 (5-year analysis)1,3
-
At IA1, KEYTRUDA demonstrated a superior PFS benefit vs platinum-based chemotherapy, with a 50% reduced risk of progression§1
- Median PFS was 10.3 months with KEYTRUDA vs 6.0 months with platinum-based chemotherapy. HR was 0.50 (95% CI: 0.37–0.68), p<0.001
5-year exploratory analysis: PFS in the ITT population3
- No statistical conclusions can be drawn from exploratory analyses
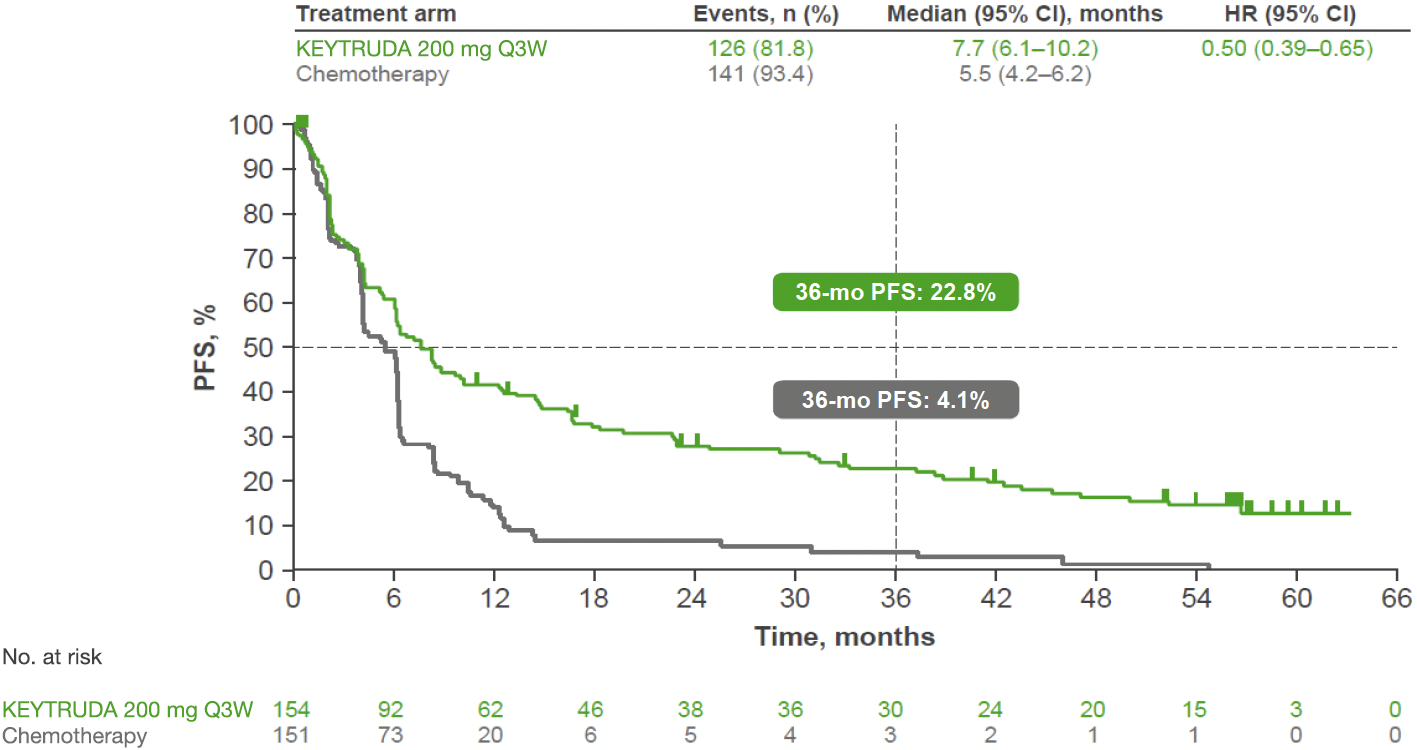
Adapted from Reck M, et al. 2021.‡3
- At the 5-year analysis, PFS benefit was sustained with KEYTRUDA vs platinum-based chemotherapy3
*5-year follow-up assessed per RECIST v1.1 by investigator review.3
†Primary endpoint was PFS assessed per blinded, independent, central radiological review.3
‡Kaplan-Meier estimate.3
§The estimated percentage of patients who were alive and had no disease progression at 6 or 12 months.1
KEYNOTE-024: OS in the ITT population (IA1 and 5-year analysis)1,3
Secondary endpoint
Median follow up: 11.2 months (IA1); 59.9 months (5-year analysis)1,3
Data cut-off date: 9 May 2016 (IA1); 1 June 2020 (5-year analysis)1,3
-
At IA1, KEYTRUDA demonstrated a superior OS benefit vs platinum-based chemotherapy, with a 40% reduced risk of death1
- Median OS was not reached with KEYTRUDA and with platinum-based chemotherapy. HR was 0.60 (95% CI: 0.41–0.89), p=0.005
- DSMC recommended stopping the trial because of superior efficacy observed with KEYTRUDA vs chemotherapy
5-year exploratory analysis: OS in the ITT population3
- No statistical conclusions can be drawn from exploratory analyses
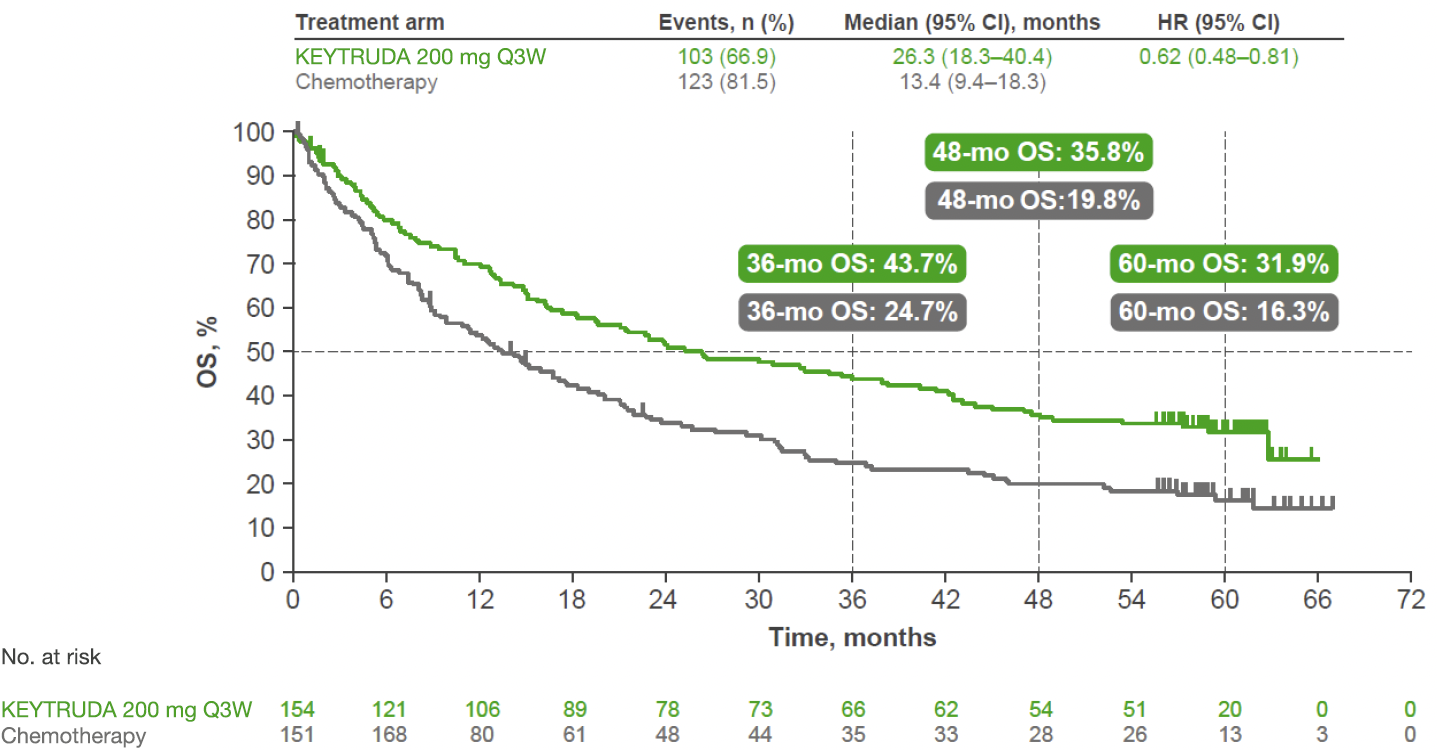
Adapted from Reck M, et al. 2021.*3
-
At the 5-year analysis:
- OS rate was numerically doubled with KEYTRUDA vs platinum-based chemotherapy3
- ORR† was 46.1% (95% CI: 38.1–54.3) with KEYTRUDA and 31.1% (95% CI: 23.8–39.2) with chemotherapy3
- Median DOR (range) was 29.1 (2.2–60.8+)‡ months with KEYTRUDA and 6.3 (3.1–52.4) months with chemotherapy3
*Kaplan-Meier estimate.3
†5-year follow-up assessed per RECIST v1.1 by investigator review.3
‡ “+” indicates response duration is censored.3
KEYNOTE-024 safety profile (as-treated population)
Summary of AEs in all treated patients (5-year update)*3
Median follow up: 11.2 months (IA1); 59.9 months (5-year analysis)1,3
Data cut-off date: 9 May 2016 (IA1); 1 June 2020 (5-year analysis)1,3
- Exposure-adjusted AE rates in the ITT population decreased over time in both treatment groups1,3
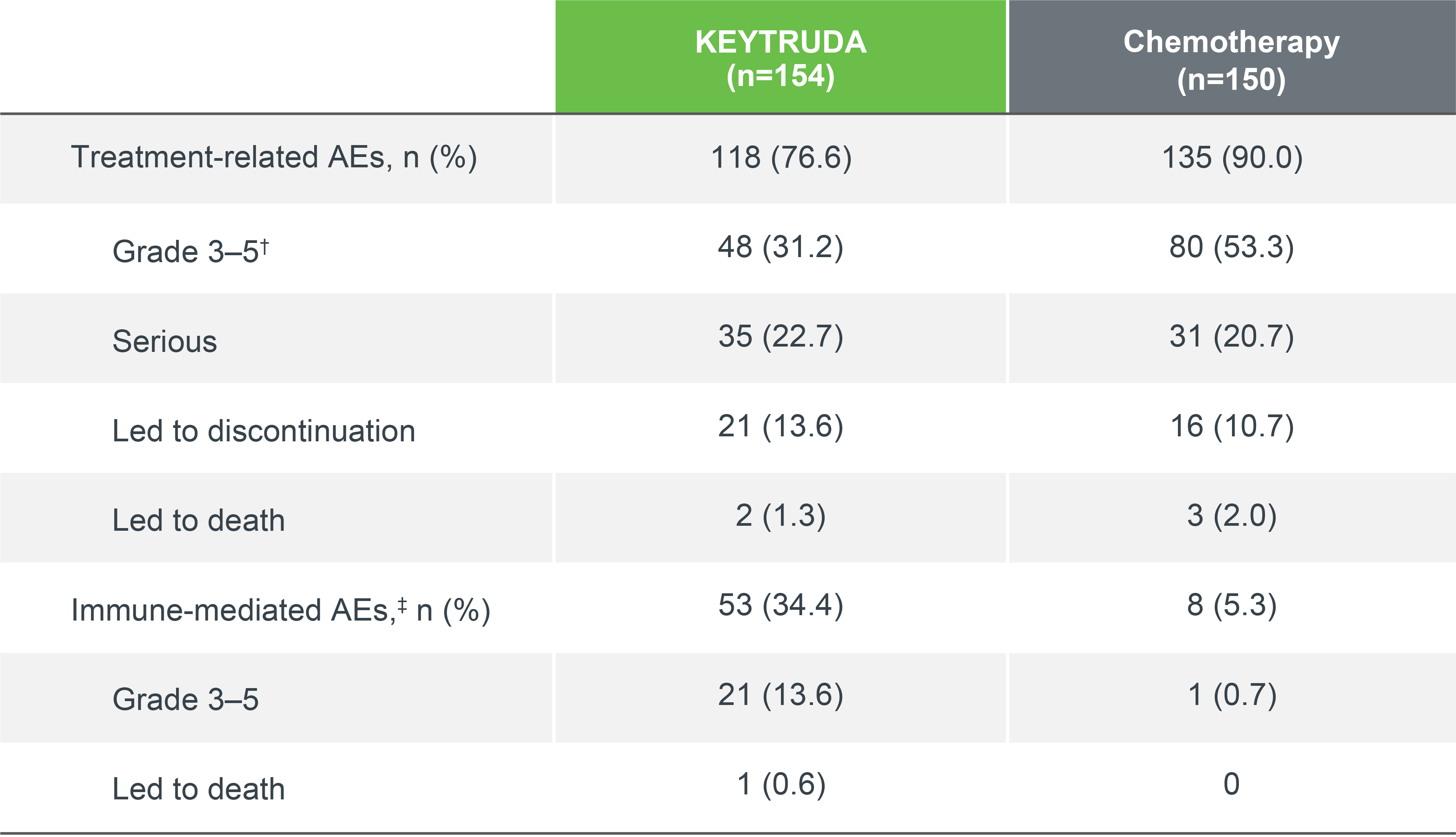
Adapted from Reck M, et al. 2021.3
-
Among patients who completed 35 cycles of KEYTRUDA (n=39), AEs were consistent with the as-treated population:3
- Treatment-related AEs occurred in 87.2% (15.4% Grade 3–4; no Grade 5 events) patients
- Immune-mediated AEs and infusion reactions occurred in 30.8% of patients (7.7% Grade 3–4; no Grade 5 events)
*During treatment with the initially assigned therapy.3
†7 additional patients in the KEYTRUDA arm and no additional patients in the chemotherapy arm had treatment-related Grade 3–5 AEs since the initial publication of KEYNOTE-024. There was no change since the updated analysis at 25.2 months median follow-up.1,6
‡Irrespective of attribution to treatment by the investigator.3
Treatment-related AEs occurring in ≥10% of patients in either group3
Median follow up: 11.2 months (IA1); 59.9 months (5-year analysis)1,3
Data cut-off date: 9 May 2016 (IA1); 1 June 2020 (5-year analysis)1,3
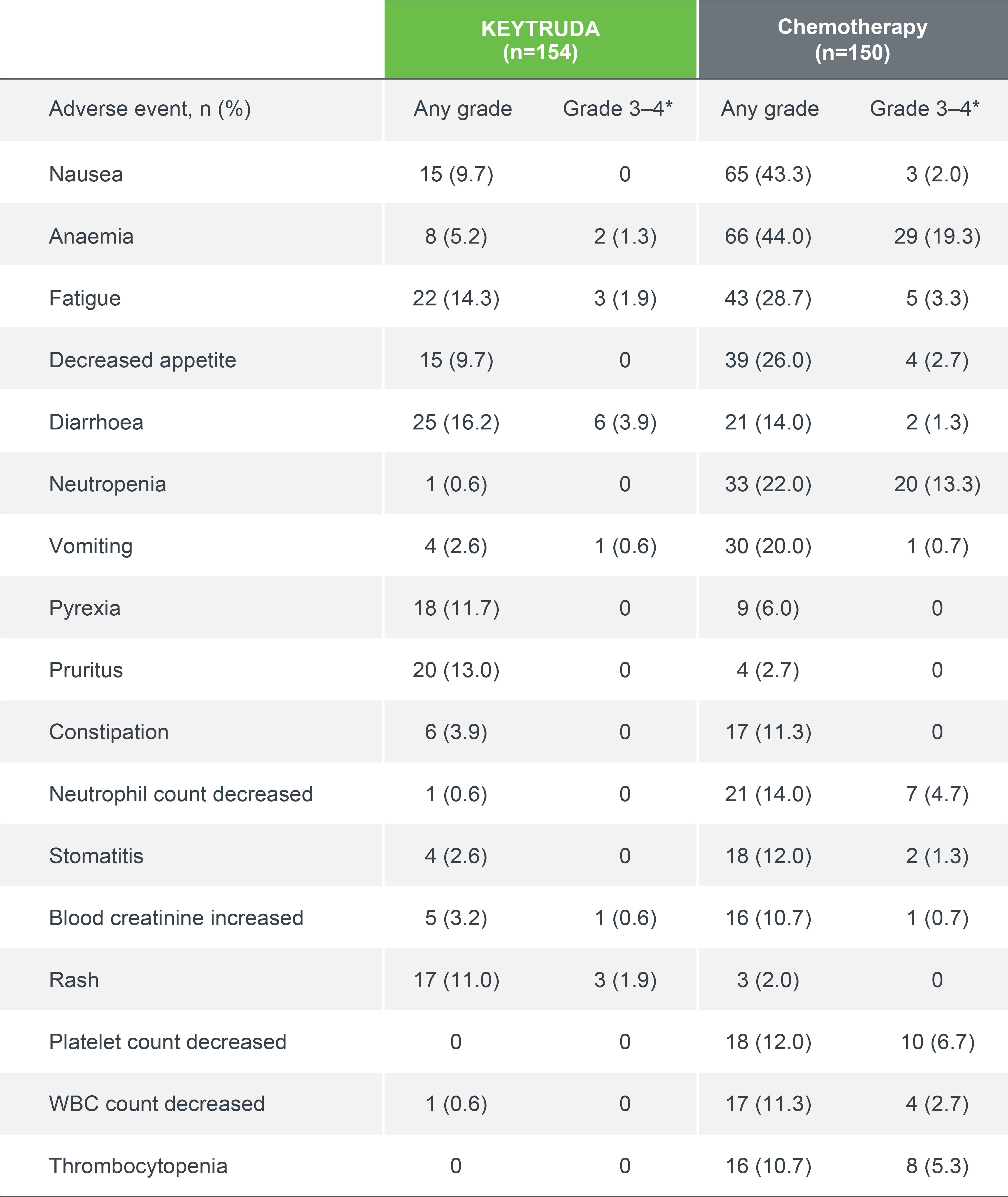
Adapted from Reck M, et al. 2021.3
*Two Grade 5 treatment-related AEs occurred in the KEYTRUDA group (pneumonitis and sudden death) and three in the chemotherapy group (pulmonary sepsis, death and pulmonary alveolar haemorrhage).3
KEYTRUDA offers flexibility of dosing2



Assessment of regimens
The 200 mg Q3W (once every 3 weeks) regimen has been assessed in phase 2 and 3 registration studies across a multitude of indications of KEYTRUDA. An exposure-response evaluation, using modelling and simulation, led to the approval of the 400 mg Q6W (once every 6 weeks) dosing for monotherapy and combination therapy.2
From a microbiological point of view, the product, once diluted, should be used immediately. The diluted solution must not be frozen. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 7 days at 2˚C to 8˚C, or 12 hours at room temperature, unless dilution has taken place in controlled and validated aseptic conditions. If refrigerated, the vials and/or intravenous bags must be allowed to come to room temperature prior to use.2
For stability related enquiries please contact medicalinformationuk@msd.com
Explore our related resources
Sign up to MSD emails
✔ Get the latest product updates
✔ Receive cancer resources
✔ Be the first to hear about our events
KEYTRUDA early-stage and advanced NSCLC indications:2
- KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with NSCLC who are at high risk of recurrence following complete resection and platinum-based chemotherapy
- KEYTRUDA, in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment, is indicated for the treatment of resectable NSCLC at high risk of recurrence in adults
- KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic NSCLC in adults whose tumours express PD-L1 with a ≥50% TPS with no EGFR– or ALK-positive tumour mutations
- KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous NSCLC in adults whose tumours have no EGFR– or ALK-positive mutations
- KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous NSCLC in adults
- KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic NSCLC in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR– or ALK-positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA
- Please consult the Summary of Product Characteristics for further information to minimise the risks associated with the use of KEYTRUDA before making prescribing decisions.
Abbreviations
AE, adverse event; ALK, anaplastic lymphoma kinase; CI, confidence interval; CR, complete response; DOR, duration of response; DSMC, data and safety monitoring committee; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; HR, hazard ratio; IA, interim analysis; ILD, interstitial lung disease; ITT, intention-to-treat; IV, intravenous; mNSCLC, metastatic non-small cell lung cancer; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PD, progressive disease; PD-L1, programmed death-ligand 1; PFS, progression-free survival; Q3W, every 3 weeks; Q6W, every 6 weeks; RECIST, Response Evaluation Criteria in Solid Tumours; TPS, tumour proportion score; WBC, white blood cell.
References
- Reck M, et al. N Engl J Med 2016;375:1823–1833.
- KEYTRUDA Summary of Product Characteristics. MSD. Available at: https://www.medicines.org.uk/emc/product/2498/smpc. Accessed: September 2025.
- Reck M, et al. J Clin Oncol 2021;39:2339–2349.
- National Institute for Health and Care Excellence. TA531: Pembrolizumab for untreated PD-L1-positive metastatic non-small-cell lung cancer. Available at: https://www.nice.org.uk/guidance/ta531. Accessed: September 2025. NICE guidance is prepared for the National Health Service in England, and is subject to regular review and may be updated or withdrawn. NICE has not checked the use of its content in this document to confirm that it accurately reflects the NICE publication from which it is taken.
- Scottish Medicines Consortium. Pembrolizumab for untreated PD-L1 positive metastatic non-small-cell lung cancer. Available at: https://scottishmedicines.org.uk/medicines-advice/pembrolizumab-keytruda-fullsubmission-123917/. Accessed: September 2025.
- Reck M, et al. J Clin Oncol 2019;37:537–546.
Supporting documentation
Prescribing Information (United Kingdom) [External link]
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website.


