KEYTRUDA plus chemotherapy in squamous mNSCLC
KEYTRUDA® (pembrolizumab) plus carboplatin-paclitaxel/nab-paclitaxel for the first-line treatment of metastatic, squamous non-small cell lung cancer1
Prescribing Information [External link]
The efficacy and safety of KEYTRUDA plus chemotherapy were assessed in previously untreated metastatic squamous NSCLC in…
KEYNOTE-407
…a randomised, multicentre, double-blind, placebo-controlled Phase III trial in 559 patients with previously untreated Stage IV metastatic squamous NSCLC1
During this trial, KEYTRUDA achieved the dual primary endpoints of OS and PFS – see below for more details.1
Licensed indication
KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel,* is indicated for the first-line treatment of metastatic squamous non-small cell lung carcinoma in adults.2
*Nab-paclitaxel is not commissioned by NHS England.3
Key eligibility criteria:
- Untreated Stage IV NSCLC with squamous histology
- ECOG PS 0 or 1
- Provision of a sample for PD-L1 assessment
Key exclusion criteria:
- Symptomatic CNS metastases
- History of non-infectious pneumonitis requiring use of glucocorticoids, active autoimmune disease and systemic immunosuppressive treatment
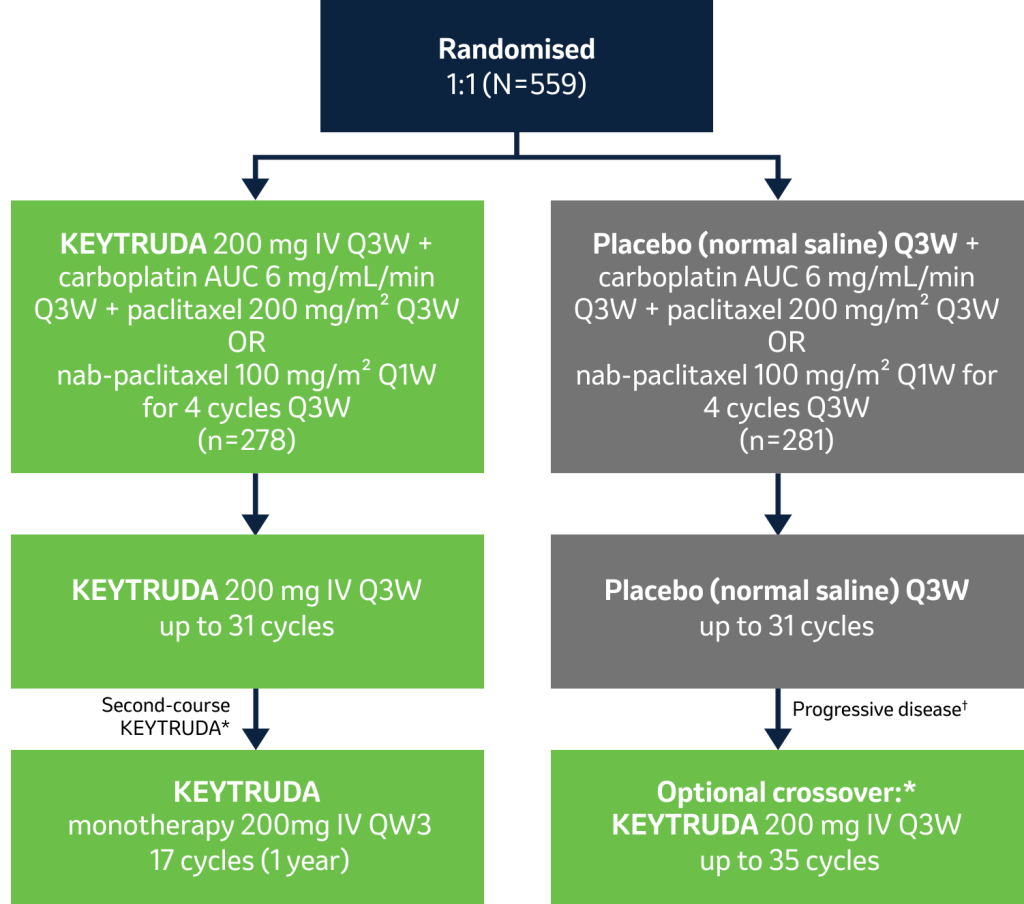
- Primary endpoints: OS, PFS‡
- Secondary endpoints: ORR,‡ DOR,‡ safety
- Exploratory endpoints: Effect of PD-L1 expression on efficacy, PROs
Stratification factors:
- PD-L1 expression (TPS§ <1% vs ≥1%)
- Choice of taxane (paclitaxel vs nab-paclitaxel)
- Geographic region (East Asia vs rest of world)
*Patients who had SD or better after 35 cycles of KEYTRUDA or stopped treatment after achieving CR and received ≥8 cycles of treatment, and received ≥2 treatments after initial CR was declared. PD could receive a second course of KEYTRUDA for 17 cycles (~ 1 year) if they had not received new cancer therapy since the last dose of KEYTRUDA.1 KEYTRUDA is not reimbursed after two years of treatment as recommended by NICE and the SMC.5,6
†Patients in the placebo arm could cross over to KEYTRUDA 200 mg Q3W during the induction or maintenance phase. To be eligible for crossover, PD must have been verified by blinded, independent, central radiological review and all safety criteria had to have been met.1
‡Assessed by blinded, independent central review per RECIST v1.1.1
§Percentage of tumour cells with membrane PD-L1 staining, as assessed using the PD-L1 IHC 22C3 pharmDx assay.1
In the original analysis, the dual primary endpoints of OS and PFS were met: KEYTRUDA plus chemotherapy (carboplatin-paclitaxel/nab-paclitaxel) provided a significant OS and PFS benefit vs carboplatin-paclitaxel/nab-paclitaxel for the first-line treatment of metastatic squamous NSCLC in adults1
KEYNOTE-407: OS in the ITT population (original analysis and 5-year analysis)*†‡1,7
Dual primary endpoint
Median follow-up: 7.8 months (original analysis); 56.9 months (5-year analysis)1,7
Data cut-off date: 3 April 2018 (original analysis); 23 February 2022 (5-year analysis)1,7
-
In the original analysis, KEYTRUDA + carboplatin + paclitaxel/nab-paclitaxel demonstrated a superior OS benefit vs carboplatin + paclitaxel/nab-paclitaxel, with a 36% reduced risk of death1,4
- Median OS was 15.9 months with KEYTRUDA + carboplatin + paclitaxel/nab-paclitaxel vs 11.3 months with carboplatin + paclitaxel/nab-paclitaxel. HR was 0.64 (95% CI: 0.49–0.85), p<0.001
5-year exploratory analysis: OS in the ITT population7
- No statistical conclusions can be drawn from exploratory analyses
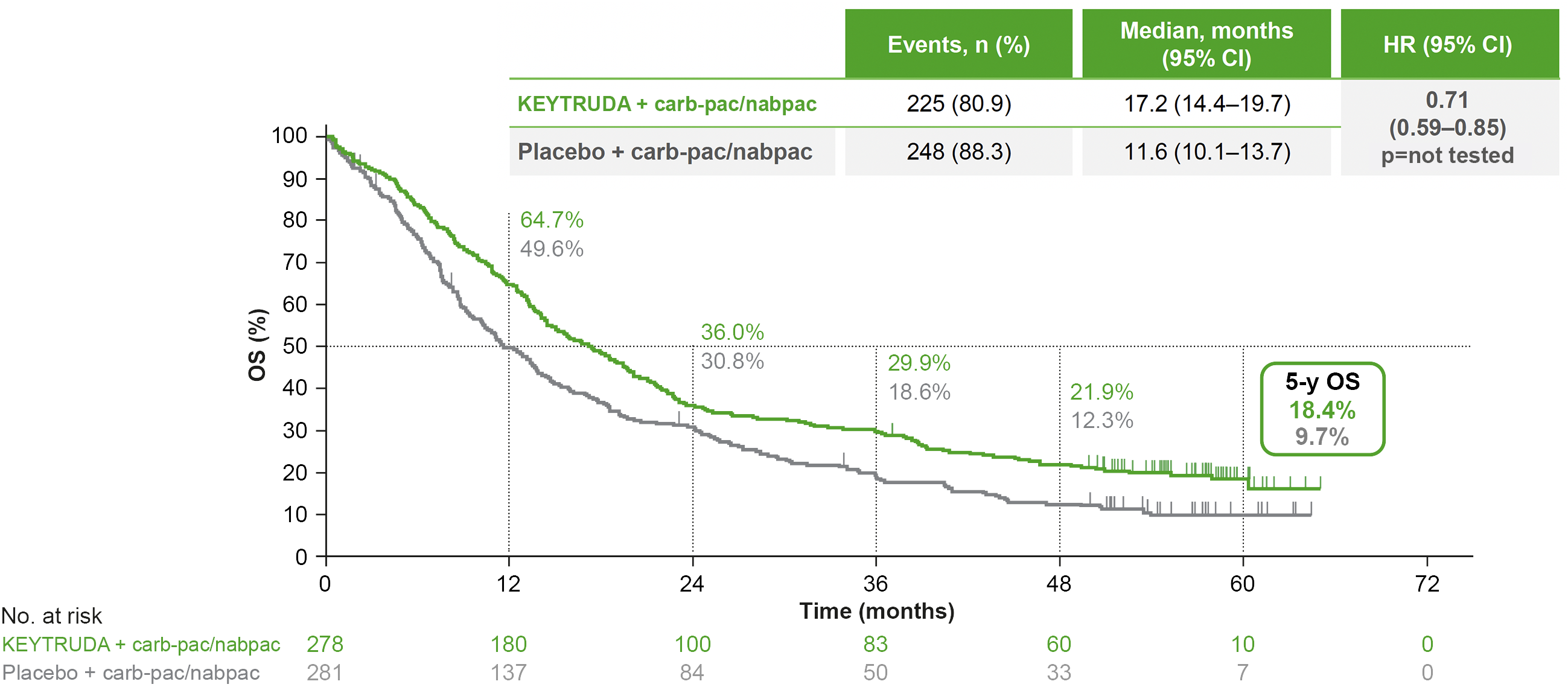
Adapted from Novello S, et al. 2023.7
*OS and PFS were both primary endpoints.7
†Kaplan-Meier estimate.7
‡Statistical significance was met for the primary endpoints in IA2 (2018).7
- At the 5-year analysis, OS benefit was sustained with KEYTRUDA + carb-pac/nabpac vs placebo + carb-pac/nabpac7
KEYNOTE-407: PFS in the ITT population (original analysis and 5-year analysis)*†1,7
Dual primary endpoint
Median follow-up: 7.8 months (original analysis); 56.9 months (5-year analysis)1,7
Data cut-off date: 3 April 2018 (original analysis); 23 February 2022 (5-year analysis)1,7
-
In the original analysis, KEYTRUDA + carboplatin + paclitaxel/nab-paclitaxel demonstrated a superior PFS benefit vs carboplatin + paclitaxel/nab-paclitaxel, with a 44% reduced risk of progression1,4
- Median PFS was 6.4 months with KEYTRUDA + carboplatin + paclitaxel/nab-paclitaxel vs 4.8 months with carboplatin + paclitaxel/nab-paclitaxel. HR was 0.56 (95% CI: 0.45–0.70), p<0.001
5-year exploratory analysis: PFS in the ITT population7
- No statistical conclusions can be drawn from exploratory analyses
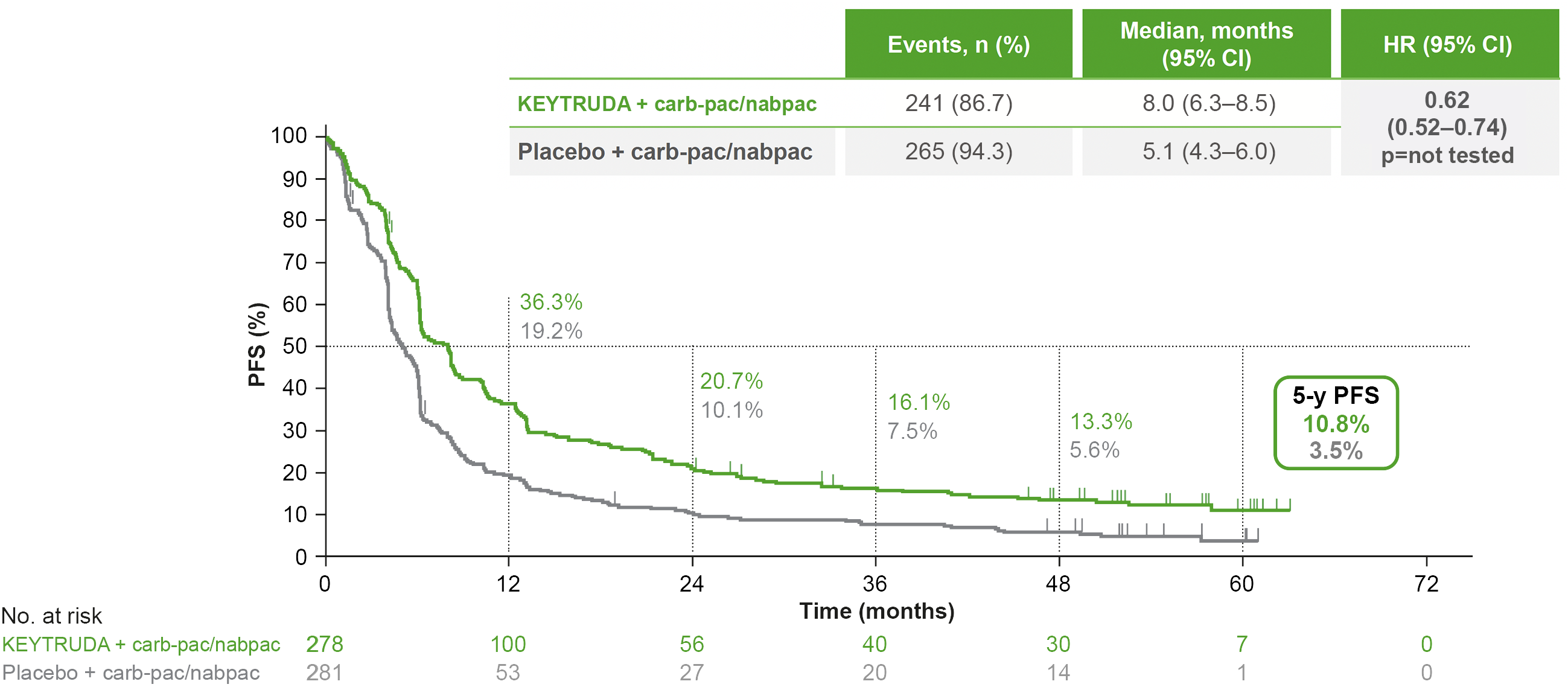
Adapted from Novello S, et al. 2023.7
*Kaplan-Meier estimate.7
†Assessed using RECIST v1.1 by blinded, independent, central review.7
-
At the 5-year analysis:
- PFS benefit was sustained with KEYTRUDA + carb-pac/nabpac vs placebo + carb-pac/nabpac7
- ORR was 62.2% (95% CI: 56.2–68.0) with KEYTRUDA + carboplatin + paclitaxel/nab+paclitaxel vs 38.8% (95% CI: 33.1–44.8) with carboplatin + paclitaxel/nab+paclitaxel7
- Median DOR (range) was 9.0 (1.3–61.5+) months with KEYTRUDA + carboplatin + paclitaxel/nab+paclitaxel vs 4.9 (1.3–58.6+) months with carboplatin + paclitaxel/nab+paclitaxel7
- A trend in survival benefit was observed in each of the PD-L1 TPS subgroups, including the PD-L1 TPS <1% expressors7
KEYNOTE-407 safety profile (as-treated population)
Summary of AEs in all treated patients (5-year update)7
Median follow-up: 56.9 months7
Data cut-off date: 23 February 20227
KEYTRUDA had a generally manageable safety profile that was comparable to carb-pac/nabpac.1,2,7 The frequency of adverse events in combination with carb-pac/nabpac was comparable to previous studies.1,2,7
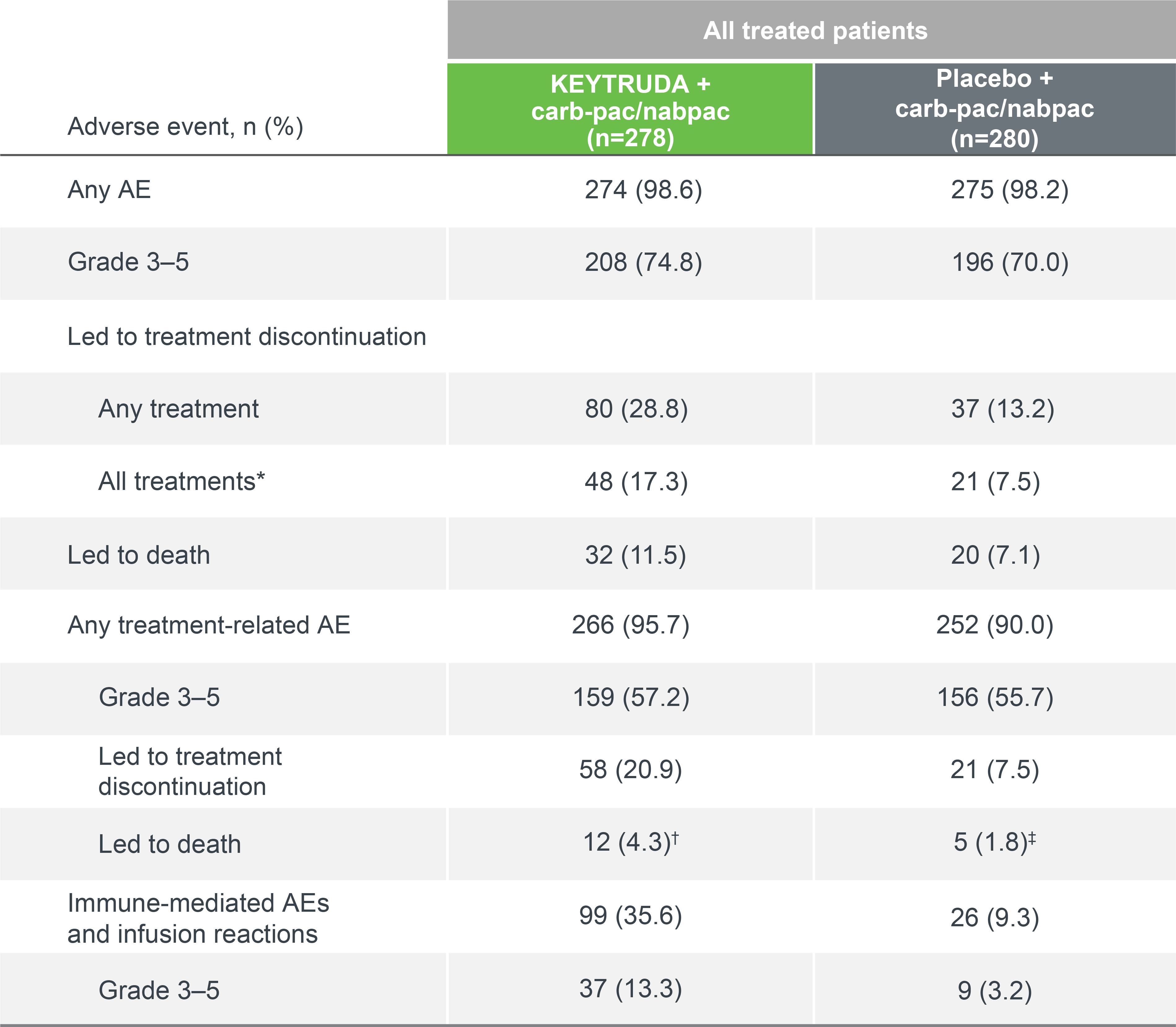
Adapted from Novello S, et al. 2023.7
Data cut-off date: 23 February 2022.7
*Includes patients who discontinued KEYTRUDA or placebo, carboplatin, and taxane owing to an AE at any time and patients who discontinued KEYTRUDA or placebo owing to an AE after completing four 3-week cycles of carboplatin and taxane.7
†Including sepsis, n=3; death (cause not specified), n=2; cardiac arrest, cardiac failure, hepatic failure, necrotising fasciitis, pneumonitis, pulmonary haemorrhage and respiratory failure, n=1 each.7
‡Including septic shock, n=2; pneumonia, acute renal injury and pulmonary haemorrhage, n=1 each.7
AEs occurring in ≥15% of patients7
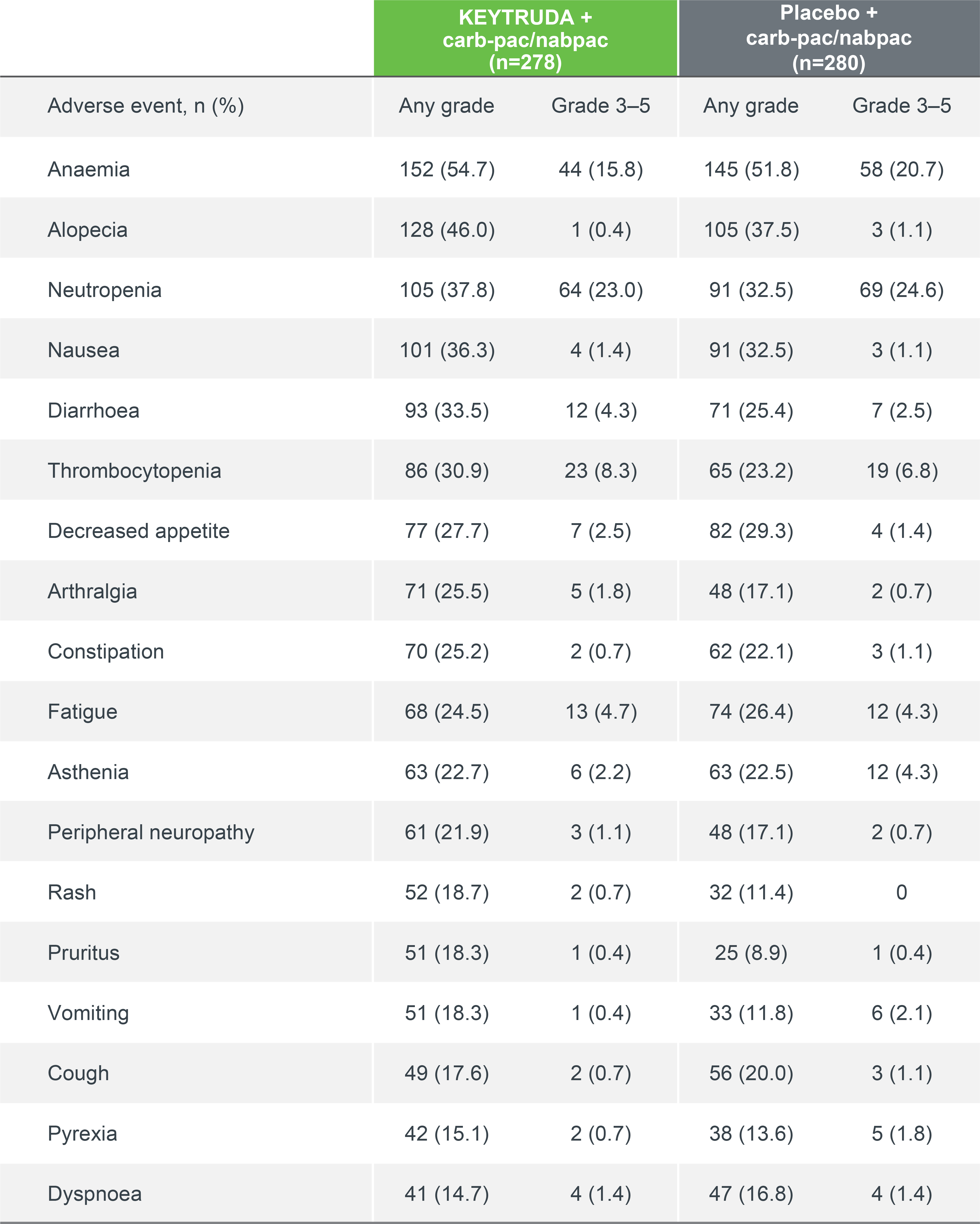
Adapted from Novello S, et al. 2023.7
KEYTRUDA offers flexibility of dosing2



Assessment of regimens
The 200 mg Q3W (once every 3 weeks) regimen has been assessed in phase 2 and 3 registration studies across a multitude of indications of KEYTRUDA. An exposure-response evaluation, using modelling and simulation, led to the approval of the 400 mg Q6W (once every 6 weeks) dosing for monotherapy and combination therapy.2
From a microbiological point of view, the product, once diluted, should be used immediately. The diluted solution must not be frozen. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 7 days at 2˚C to 8˚C, or 12 hours at room temperature, unless dilution has taken place in controlled and validated aseptic conditions. If refrigerated, the vials and/or intravenous bags must be allowed to come to room temperature prior to use.2
For stability related enquiries please contact medicalinformationuk@msd.com
Explore our related resources
Sign up to MSD emails
✔ Get the latest product updates
✔ Receive cancer resources
✔ Be the first to hear about our events
KEYTRUDA early-stage and advanced NSCLC indications:2
- KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with NSCLC who are at high risk of recurrence following complete resection and platinum-based chemotherapy
- KEYTRUDA, in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment, is indicated for the treatment of resectable NSCLC at high risk of recurrence in adults
- KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic NSCLC in adults whose tumours express PD-L1 with a ≥50% TPS with no EGFR– or ALK-positive tumour mutations
- KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous NSCLC in adults whose tumours have no EGFR– or ALK-positive mutations
- KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous NSCLC in adults
- KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic NSCLC in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR– or ALK-positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA
- Please consult the Summary of Product Characteristics for further information to minimise the risks associated with the use of KEYTRUDA before making prescribing decisions.
Abbreviations
AE, adverse event; ALK, anaplastic lymphoma kinase; AUC, area under the curve; carb-pac/nabpac, carboplatin + paclitaxel or nab-paclitaxel; CI, confidence interval; CNS, central nervous system; DOR, duration of response; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; HR, hazard ratio; IA, interim analysis; IHC, immunohistochemistry; ITT, intent to treat; IV, intravenous; NSCLC, non-small cell lung cancer; ORR, overall response rate; OS, overall survival; PD, progressive disease; PD-L1, programmed death-ligand 1; PFS, progression free survival; PRO, patient-reported outcome; Q1W, every 1 week; Q3W, every 3 weeks; Q6W, every 6 weeks; RECIST, Response Evaluation Criteria in Solid Tumours; TPS, tumour proportion score.
References
- Paz-Ares L, et al. N Engl J Med 2018;379:2040–2051 (and protocol).
-
KEYTRUDA Summary of Product Characteristics. MSD.
Available at: https://www.medicines.org.uk/emc/product/2498/smpc. Accessed: September 2025. - National Institute for Health and Care Excellence. Final appraisal document: Pembrolizumab with carboplatin and paclitaxel for untreated metastatic squamous non-smallcell lung cancer Available at: https://www.nice.org.uk/guidance/ta770/documents/final-appraisal- determination-document-2. Accessed: September 2025.
- Paz-Ares L, et al. KEYNOTE-407: Phase 3 study of carboplatin-paclitaxel/nab-paclitaxel with or without pembrolizumab for metastatic squamous NSCLC. ASCO. 1–5 June 2018. Chicago, USA.
- National Institute for Health and Care Excellence. TA770: Pembrolizumab with carboplatin and paclitaxel for untreated metastatic squamous non-smallcell lung cancer. Available at: https://www.nice.org.uk/guidance/ta770. Accessed: September 2025. NICE guidance is prepared for the National Health Service in England, and is subject to regular review and may be updated or withdrawn. NICE has not checked the use of its content in this document to confirm that it accurately reflects the NICE publication from which it is taken.
- Scottish Medicines Consortium. Appraisal determination: Pembrolizumab 25mg/mL concentrate for solution for infusion and 50mg powder for concentrate for solution for infusion (KEYTRUDA®). Available at: https://www.scottishmedicines.org.uk/medicines-advice/pembrolizumab-keytruda-full-smc2187/. Accessed: September 2025.
- Novello S, et al. J Clin Oncol 2023;41:1999–2006.
Supporting documentation
Prescribing Information (United Kingdom) [External link]
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website.


