KEYTRUDA plus KISPLYX in first-line
KEYTRUDA® (pembrolizumab) Prescribing Information for Great Britain & Northern Ireland [External links]
KISPLYX® (lenvatinib) Prescribing information for Great Britain & Northern Ireland [External link]
The recommended dose of KEYTRUDA in adults is either 200 mg every 3 weeks or 400 mg every 6 weeks, administered as an intravenous infusion over 30 minutes.1
The recommended dose of KISPLYX is 20 mg QD, at the same time each day administered PO, with or without food, swallowed whole with water. For patients unable to swallow capsules, please refer to the SmPC for alternative methods of preparation.2
KEYTRUDA + KISPLYX has a dual MOA, inhibiting two disease pathways3–6
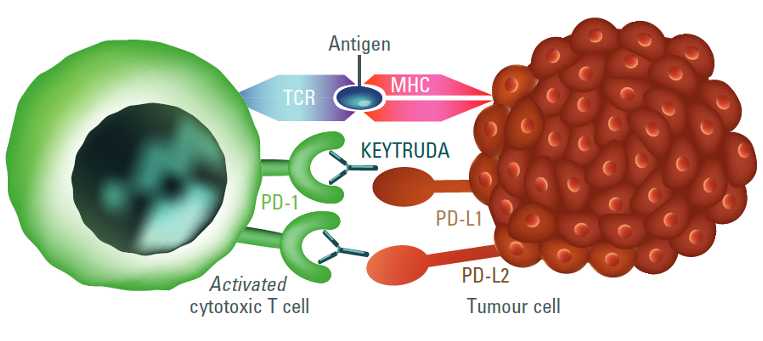
The immune-stimulatory effect of KEYTRUDA (anti-PD-1)1,7
- KEYTRUDA is a selective, humanised, monoclonal antibody designed to block the interaction between PD-1 and its ligands, PD-L1 and PD-L21
- By inhibiting PD-1 receptor binding, KEYTRUDA reactivates tumour-specific cytotoxic T lymphocytes in the tumour microenvironment, resulting in anti-tumour immunity1
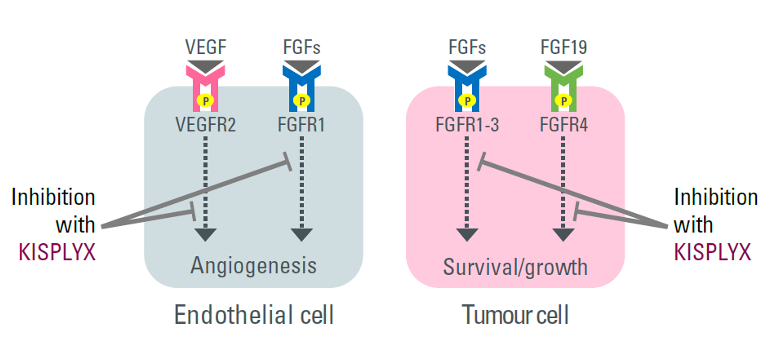
The anti-angiogenic effect of KISPLYX (anti-VEGF/FGF)2,8
- KISPLYX is a RTK inhibitor that selectively inhibits the kinase activities of VEGF receptors, VEGFR1 (FLT1), VEGFR2 (KDR), and VEGFR3 (FLT4), in addition to other proangiogenic and oncogenic pathway-related RTKs including FGF receptors FGFR1, 2, 3, and 4; the PDGF receptor, PDGFRα; KIT; and RET2,9
Figures adapted from Pardoll DM, 2012 (left) and Kudo M, 2018 (right).7,8
The anti-angiogenic effect of KISPLYX (multi-TKI) in combination with the immuno-stimulatory effect of KEYTRUDA (anti–PD-1) results in a tumour microenvironment with greater T-cell activation to help overcome primary and acquired resistance to immunotherapy and may improve tumour responses compared to either treatment alone in pre-clinical models.1
The efficacy and safety of KEYTRUDA + KISPLYX vs sunitinib monotherapy were investigated in the CLEAR trial
A randomised, multicentre, open-label, Phase III trial evaluating the efficacy and safety of KEYTRUDA + KISPLYX in patients with advanced RCC in the 1L setting (N=1069)
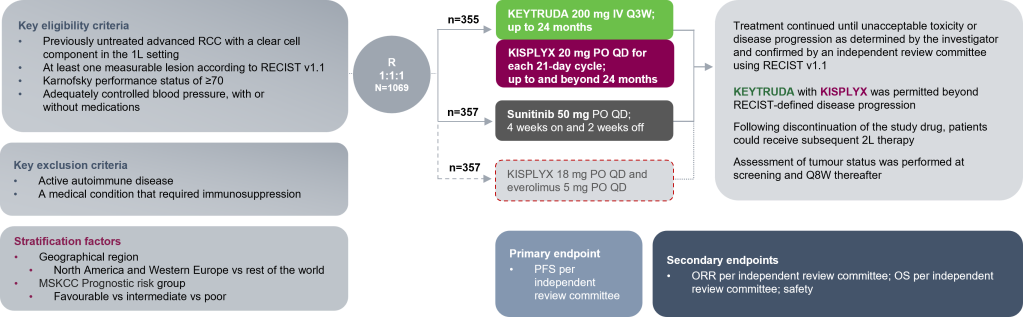
Adapted from Motzer R et al. N Engl J Med. 2021;384(14);1289‒1300.
1L use of KISPLYX in combination with everolimus is not approved in the UK in patients with advanced RCC. This treatment arm has been included for transparency. Clinical data shown is from the KEYTRUDA + KISPLYX versus sunitinib arms only.1,2
Full eligibility and exclusion criteria are described in the trial protocol.
Patient baseline demographics and disease characteristics10
| Characteristics* n (%) | KEYTRUDA + KISPLYX (n=355) | Sunitinib (n=357) |
|---|---|---|
| Median age (range), years | 64 (34–88) | 61 (29–82) |
| Aged <65 years | 194 (54.6) | 225 (63.0) |
| Sex | ||
| Male | 255 (71.8) | 275 (77.0) |
| Female | 100 (28.2) | 82 (23.0) |
| Geographic region | ||
| Western Europe or North America | 198 (55.8) | 199 (55.7) |
| Rest of the World | 157 (44.2) | 158 (44.3) |
| Karnofsky performance status† | ||
| 100–90 | 295 (83.1) | 294 (82.4) |
| 80–70 | 60 (16.9) | 62 (17.4) |
| MSKCC prognostic risk group | ||
| Favourable | 96 (27.0) | 97 (27.2) |
| Intermediate | 227 (63.9) | 228 (63.9) |
| Poor | 32 (9.0) | 32 (9.0) |
| IMDC prognostic risk group | ||
| Favourable | 110 (31.0) | 124 (34.7) |
| Intermediate | 210 (59.2) | 192 (53.8) |
| Poor | 33 (9.3) | 37 (10.4) |
| Could not be evaluated | 2 (0.6) | 4 (1.1) |
| Sarcomatoid features | 28 (7.9) | 21 (5.9) |
| PD-L1 combined positive score | ||
| ≥1 | 107 (30.1) | 119 (33.3) |
| <1 | 112 (31.5) | 103 (28.9) |
| Not available | 136 (38.3) | 135 (37.8) |
| No. of metastatic organs or sites‡ | ||
| 1 | 97 (27.3) | 108 (30.3) |
| ≥2 | 254 (71.5) | 246 (68.9) |
| Site of metastasis§ | ||
| Lung | 249 (70.1) | 239 (66.9) |
| Lymph node | 170 (47.9) | 159 (44.5) |
| Bone | 85 (23.9) | 97 (27.2) |
| Liver | 60 (16.9) | 61 (17.1) |
| Previous nephrectomy | 262 (73.8) | 275 (77.0) |
Adapted from Motzer R et al. N Engl J Med. 2021;384(14);1289‒1300.
*One patient in the KEYTRUDA + KISPLYX group has carcinoma without a clear cell component;
†Karnofsky performance status score was missing for one patient in the sunitinib group;
‡Kidney was not included in the number of metastatic organs or site;
§Four common sites of metastasis are shown. Patients may have had metastasis at more than one site.
KEYTRUDA + KISPLYX demonstrated superior PFS, ORR and OS vs. sunitinib in the 1L treatment of advanced RCC1,2
KEYTRUDA + KISPLYX more than doubled median PFS vs sunitinib10*
PFS was significantly longer in the KEYTRUDA + KISPLYX group compared with the sunitinib group
Primary endpoint

Adapted from Motzer R et al. N Engl J Med. 2021;384(14);1289‒1300.
Analysis cutoff date: 28 August 2020. Median follow up 26.6 months.
*Assessed using RECIST v1.1 by an independent review committee.
Superior OS with KEYTRUDA + KISPLYX vs. sunitinib10
Secondary endpoint
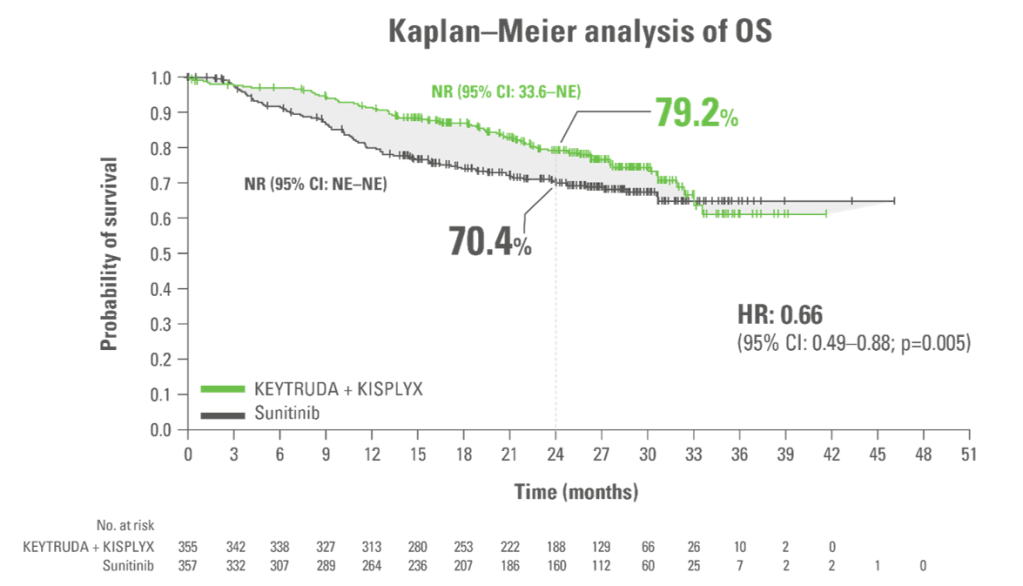
Adapted from Motzer R et al. N Engl J Med. 2021;384(14);1289‒1300.
KEYTRUDA + KISPLYX superior OS vs sunitinib: Reduced the risk of death by 34% (HR:* 0.66; 95% CI: 0.49–0.88; p=0.005†)
- Median OS: NR in both arms
Analysis cutoff date: 28 August 2020. Median follow up 26.6 months.
*Based on the stratified Cox proportional hazard model.
†Two sided p-value based on stratified log-rank test.
34 month exploratory OS analysis consistent with primary analysis11,12*
Secondary endpoint
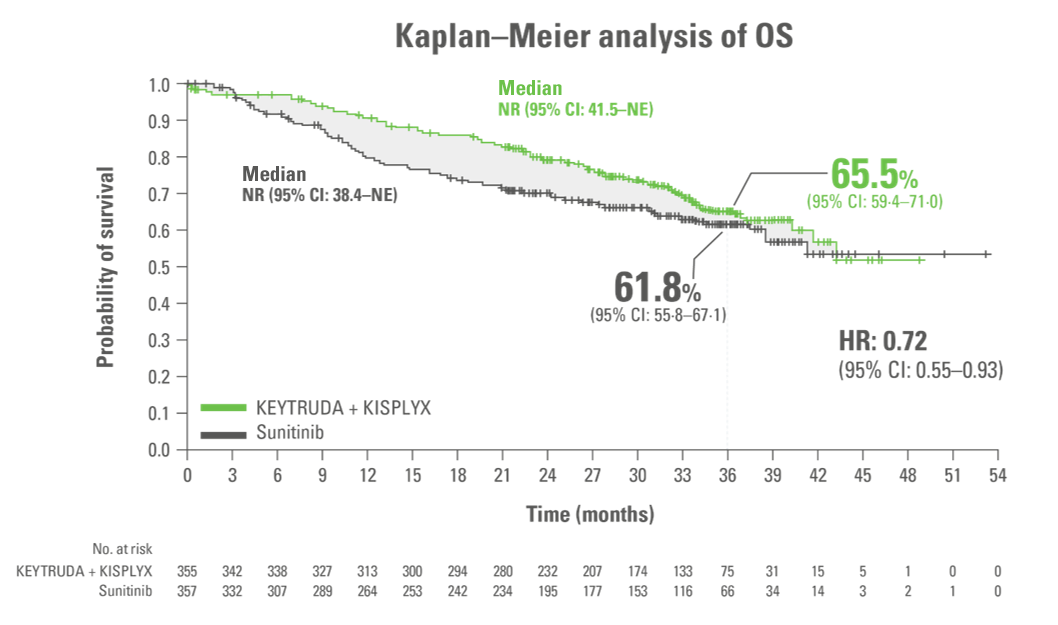
Adapted from Choueiri TK et al. 2023.
LIMITATION
This 34-month extension analysis was an exploratory analysis.
No formal statistical testing was performed for this analysis, and, therefore, no conclusions can be drawn.
- Median OS: NR in both arms11
Analysis cutoff date: 31 March 2021. Median duration of follow-up for OS: 33.7 months (95% CI: 32.8–34.4) with KEYTRUDA + KISPLYX vs 33.4 months (95% CI: 32.5–34.1) with sunitinib.11,12
*Of patients in the KEYTRUDA + KISPLYX and sunitinib arms, 250 (70.4%) and 235 (65.8%) were censored, respectively.
†Overall survival rates at 12, 18 and 24 months were 91.4%, 86.9% and 80.2% for KEYTRUDA + KISPLYX and 80.2%, 73.8% and 69.7% for sunitinib.
ORR nearly double with KEYTRUDA + KISPLYX vs sunitinib1,2,10
Secondary endpoint
Superior ORR vs sunitinib
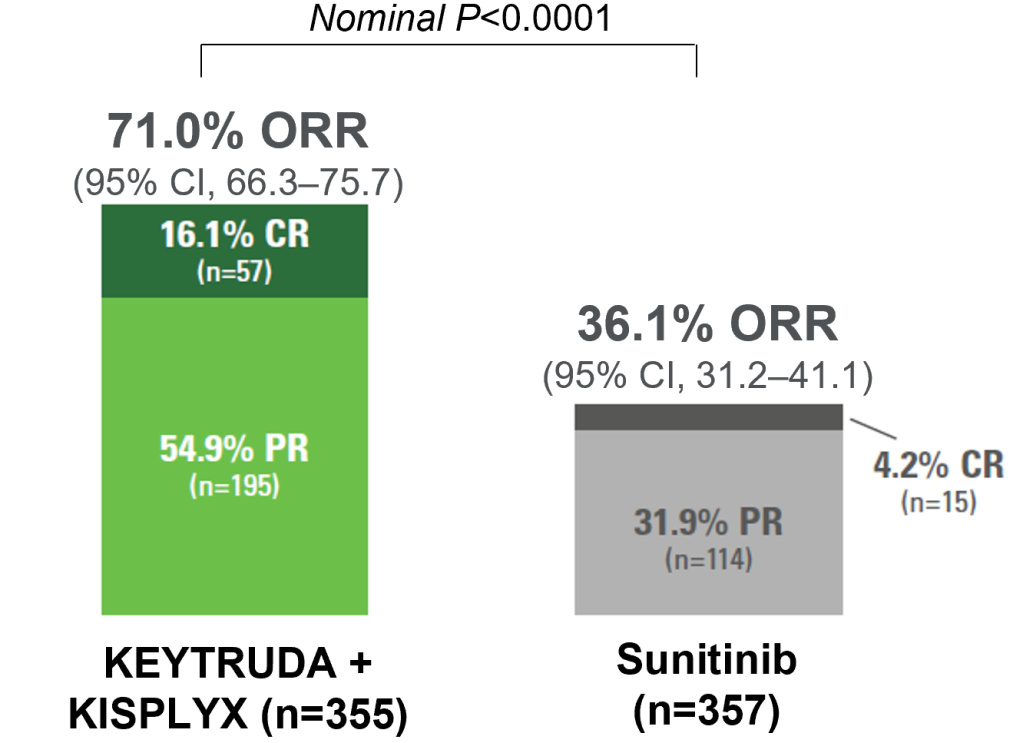
Adapted from Motzer R et al. N Engl J Med. 2021;384(14);1289‒1300.
- Progressive disease was observed in 5.4% of patients with KEYTRUDA + KISPLYX compared with 14% of patients with sunitinib
- Stable disease was observed in 19.2% of patients with KEYTRUDA + KISPLYX compared with 38.1% of patients with sunitinib
Analysis cutoff date: 28 August 2020. Median follow up 26.6 months.
Median DoR was 25.8 months for KEYTRUDA + KISPLYX and 14.6 months for sunitinib10*
Exploratory endpoint
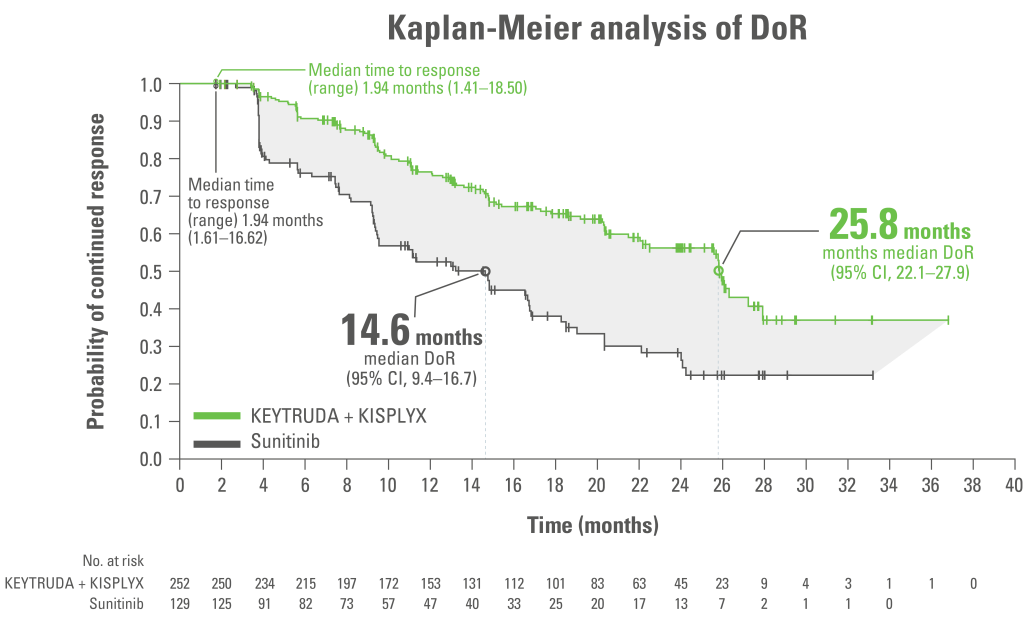
Adapted from Motzer R et al. N Engl J Med. 2021;384(14);1289‒1300.
LIMITATION: This analysis was a protocol pre-specified descriptive analysis. No formal statistical analysis was performed for this analysis; therefore, no conclusions can be drawn.
Analysis cutoff date: 28 August 2020. Median follow up 26.6 months.
*Responses were assessed by an IRC using RECIST 1.1.
AE summary for KEYTRUDA + KISPLYX compared with sunitinib10*
The most common all grade adverse events in the KEYTRUDA + KISPLYX arm were diarrhoea (61.4%), hypertension (55.4%) and hypothyroidism (47.2%).
Secondary endpoint
| Event | KEYTRUDA + KISPLYX (n=352) | Sunitinib (n=340) |
|---|---|---|
| Median duration of treatment, months (range) | 17.0 (0.1–39.1) | 7.8 (0.1–37.0) |
| AE of any grade, n (%) | 351 (99.7) | 335 (98.5) |
| Grade ≥3 AE, n (%) | 290 (82.4) | 244 (71.8) |
| Death during treatment (Grade 5 AE), n (%)† | 15 (4.3) | 11 (3.2) |
| Discontinuation due any grade AE, % | 37.2 | 14.4 |
| KEYTRUDA only, % | 28.7 | – |
| KISPLYX only, % | 25.6 | – |
| Both drugs, % | 13.4 | – |
| Dose reduction due to any grade AE, %‡ | 68.8 | 50.3 |
| Interruption of treatment due to any grade AE, % | 78.4 | 53.8 |
Adapted from Motzer R et al. N Engl J Med. 2021;384(14);1289‒1300.
Analysis cutoff date: 28 August 2020. Median follow up 26.6 months.
*Safety assessment was based on as treated principle and consisted of monitoring and recording of all AEs and serious AEs using the Common Terminology Criteria for Adverse Events, Version 4.03, in the group of patients who received at least one dose of the trial drug.
†Of the 15 patients in the KEYTRUDA + KISPLYX group who had grade 5 AEs during treatment, 11 had fatal AEs not attributed to disease progression (acute renal failure, uncontrolled hypertension, complications from myasthenic syndrome, complications from autoimmune hepatitis, cardiac arrest, and death–cause not specified in 1 patient each; haemorrhagic events in 2 patients; and sepsis in 3 patients). Among the 11 patients in the sunitinib group with grade 5 AEs during treatment, fatal AEs not attributed to disease progression occurred in 2 patients (respiratory failure and acute kidney injury in 1 patient and death–cause not specified in 1 patient);
‡Dose reduction in KISPLYX only. Dose reductions for KEYTRUDA are not recommended.
For further information about TEAE please refer to the SmPC.
AEs of any cause that emerged or worsened during treatment in ≥25% of patients in either treatment group10*
Secondary endpoint
AEs of any cause that emerged or worsened during treatment in ≥25% of patients of either treatment group during treatment with KEYTRUDA + KISPLYX vs. sunitinib
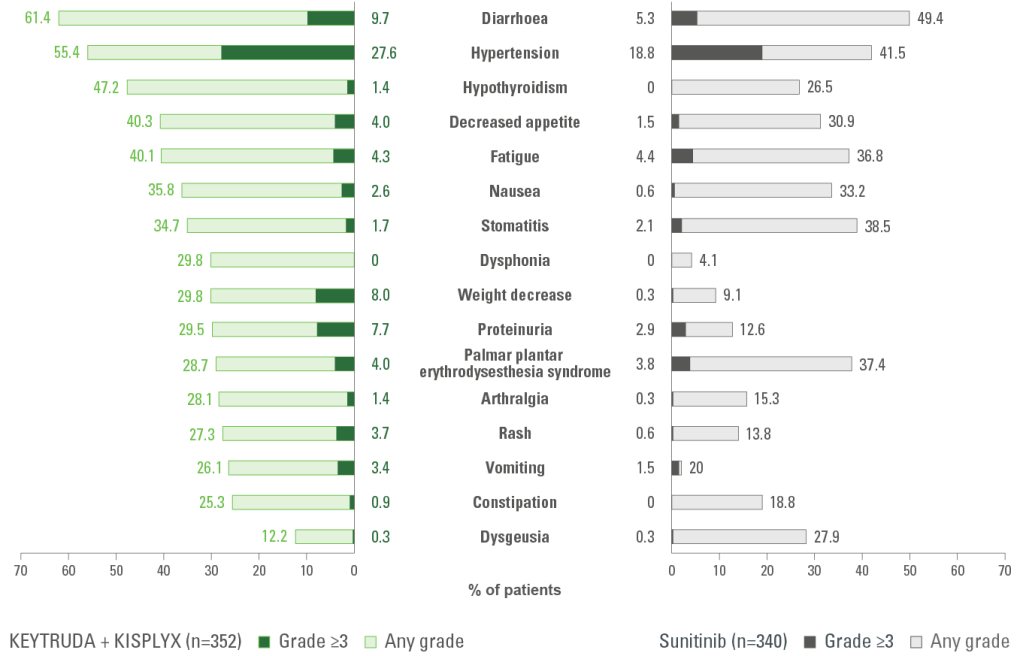
Adapted from Motzer R et al. N Engl J Med. 2021;384(14);1289‒1300.
Analysis cutoff date: 28 August 2020.
Safety assessment was based on an as-treated principle and consisted of monitoring and recording all AEs and serious AEs using the Common Terminology Criteria for AEs, version 4.03, in the group of patients who received at least one dose of the study drug. Hypothyroidism is an AE of interest associated with KEYTRUDA.
Please refer to the SmPC for a full overview of the AEs associated with KEYTRUDA.
Summary of TEAEs of interest for KEYTRUDAa,10
Secondary endpoint
| TEAE | KEYTRUDA + KISPLYX (n=352) | KEYTRUDA + KISPLYX (n=352) | Sunitinib (n=340) | Sunitinib (n=340) |
|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Any | 214 (60.8) | 52 (14.8) | 105 (30.9) | 4 (1.2) |
| Adrenal insufficiency | 18 (5.1) | 4 (1.1) | 0 (0.0) | 0 (0.0) |
| Colitis | 9 (2.6) | 4 (1.1) | 2 (0.6) | 0 (0.0) |
| Encephalitis | 2 (0.6) | 2 (0.6) | 0 (0.0) | 0 (0.0) |
| Hepatitis | 7 (2.0) | 5 (1.4) | 0 (0.0) | 0 (0.0) |
| Hyperthyroidism | 28 (8.0) | 0 (0.0) | 12 (3.5) | 0 (0.0) |
| Hypophysitis | 3 (0.9) | 2 (0.6) | 0 (0.0) | 0 (0.0) |
| Hypothyroidism | 166 (47.2) | 5 (1.4) | 90 (26.5) | 0 (0.0) |
| Infusion reactions | 5 (1.4) | 1 (0.3) | 2 (0.6) | 0 (0.0) |
| Myasthenic syndrome | 1 (0.3) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| Myocarditis | 4 (1.1) | 3 (0.9) | 0 (0.0) | 0 (0.0) |
| Myositis | 3 (0.9) | 2 (0.6) | 0 (0.0) | 0 (0.0) |
| Nephritis | 6 (1.7) | 4 (1.1) | 0 (0.0) | 0 (0.0) |
| Pancreatitis | 10 (2.8) | 6 (1.7) | 2 (0.6) | 1 (0.3) |
| Pneumonitis | 19 (5.4) | 7 (2.0) | 0 (0.0) | 0 (0.0) |
| Severe skin reactions | 18 (5.1) | 18 (5.1) | 5 (1.5) | 3 (0.9) |
| Thyroiditis | 2 (0.6) | 0 (0.0) | 2 (0.6) | 0 (0.0) |
| Type 1 diabetes mellitus | 2 (0.6) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| Uveitis | 1 (0.3) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
Adapted from Motzer et al. 202110
Analysis cutoff date: 28 August 2020.
aNo cases of Guillain-Barré syndrome, myelitis, or sarcoidosis were reported in any group.
AE, adverse event, TEAE, treatment-emergent adverse event..
Summary of clinically significant TEAEs for KISPLYX10
Secondary endpoint
| TEAE | KEYTRUDA + KISPLYX (n=352) | KEYTRUDA + KISPLYX (n=352) | Sunitinib (n=340) | Sunitinib (n=340) |
|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Any | 331 (94.0) | 188 (53.4) | 289 (85.0) | 118 (34.7) |
| Arterial thromboembolic events | 19 (5.4) | 13 (3.7) | 7 (2.1) | 2 (0.6) |
| Cardiac dysfunction | 9 (2.6) | 6 (1.7) | 7 (2.1) | 4 (1.2) |
| Fistula formation | 2 (0.6) | 0 (0.0) | 2 (0.6) | 1 (0.3) |
| Gastrointestinal perforation | 5 (1.4) | 4 (1.1) | 3 (0.9) | 1 (0.3) |
| Haemorrhage | 96 (27.3) | 18 (5.1) | 90 (26.5) | 13 (3.8) |
| Hepatotoxicity | 96 (27.3) | 35 (9.9) | 82 (24.1) | 18 (5.3) |
| Hypertension | 198 (56.3) | 101 (28.7) | 145 (42.6) | 66 (19.4) |
| Hypocalcaemia | 5 (1.4) | 1 (0.3) | 9 (2.6) | 1 (0.3) |
| Hypothyroidism | 200 (56.8) | 5 (1.4) | 109 (32.1) | 0 (0.0) |
| Palmar-Plantar erythodysesthesia syndrome | 104 (29.5) | 14 (4.0) | 129 (37.9) | 13 (3.8) |
| Posterior reversible encephalopathy syndrome | 2 (0.6) | 2 (0.6) | 1 (0.3) | 0 (0.0) |
| Proteinuira | 104 (29.5) | 27 (7.7) | 43 (12.6) | 10 (2.9) |
| QT prolongation | 23 (6.5) | 10 (2.8) | 13 (3.8) | 4 (1.2) |
| Renal events | 78 (22.2) | 20 (5.7) | 60 (17.6) | 8 (2.4) |
Adapted from Motzer et al. 202110
Analysis cutoff date: 28 August 2020.
AE, adverse event, TEAE, treatment-emergent adverse event
Study summary10
Efficacy and safety data from the CLEAR trial supports the use of KEYTRUDA + KISPLYX as a 1L treatment option in patients with advanced RCC11
PFS
PFS more than doubled with KEYTRUDA + KISPLYX vs. sunitinib
- 23.9 months vs. 9.2 months, respectively (HR 0.39; 95% CI, 0.32–0.49; P<0.001)
ORR
KEYTRUDA + KISPLYX almost doubled the ORR vs. sunitinib
ORR: 71% vs. 36.1%
CR: 16.1% vs. 4.2%
PR: 54.9% vs. 31.9%
OS
Superior OS with KEYTRUDA + KISPLYX vs. sunitinib
34% relative risk reduction of death with KEYTRUDA + KISPLYX vs. sunitinib (HR [95% CI]: 0.66 [0.49,0.88]; P=0.005)
Absolute risk: Events observed were 23% (80/355) with KEYTRUDA+ KISPLYX vs. 28% (101/357) with sunitinib
The safety profile of KEYTRUDA + KISPLYX was consistent with the known profile of each drug, as well as previously reported safety profiles for the combination
Consider KEYTRUDA + KISPLYX as a 1L treatment option in eligible patients with advanced RCC
Dosing1,2
KEYTRUDA and KISPLYX are administered via IV infusion and oral capsules, respectively1,2
The 200 mg Q3W (once every 3 weeks) regimen has been assessed in Phase 2 and 3 registration studies across a multitude of indications of KEYTRUDA. An exposure-response evaluation, using modelling and simulation, led to the approval of the 400 mg Q6W (once every 6 weeks) dosing for monotherapy and combination therapy1

- Continue treatment with KISPLYX for as long as there is clinical benefit or until unacceptable toxicity occurs
- For AEs thought to be related to KISPLYX, upon resolution/improvement of an AE to Grade 0–1 or baseline, treatment should be resumed at a reduced dose of KISPLYX
- Please refer to the KISPLYX SmPC for the management of AEs
- Please refer to the SmPC for information on dose modifications in combination with KEYTRUDA
Abbreviations
1L = First Line; 2L = Second Line; AE = Adverse Event; CI = Confidence Interval; CR = Complete Response; DoR = Duration of Response; FGF = Fibroblast Growth Factor; FGFR = Fibroblast Growth Factor Receptor; FLT1 = FMS-like Tyrosine Kinase 1; FLT4 = FMS-like Tyrosine Kinase 4; HR = Hazard Ratio; HRQoL = Health Related Quality of Life; IMDC = International Metastatic RCC Database Consortium; IO = Immuno-oncology; irAE = Immune-related Adverse Event; IRC = Independent Review Committee; irRECIST = Immune-related Response Evaluation Criteria In Solid Tumors; ITT = Intention To Treat; IV = intravenous; KIT = proto-oncogene c-KIT; KDR = Kinase Insert Domain Receptor; LEN = Lenvatinib; MHC = Major Histocompatibility Complex; MOA = Mode Of Action; MSKCC = Memorial Sloan Kettering Cancer Center; NE = Not Estimable; NR = Not Reached; ORR = Objective Response Rate; OS = Overall Survival; PD-1 = Programmed Death Receptor-1; PDGFR = Platelet-Derived Growth Factor; PD-L1 = Programmed Death Ligand 1; PD-L2 = Programmed Death Ligand-2; PEM = Pembrolizumab; PFS = Progression Free Survival; PFS2 = PFS On Next Line Therapy; PK = Pharmacokinetics; PO = Orally; PR = Partial Response; Q3W = Every 3 Weeks; Q8W = Every 8 Weeks; QD = once daily; R = Randomisation; RCC = Renal Cell Carcinoma; RECIST v1.1 = Response Evaluation Criteria in Solid Tumors Version 1.1; RET = proto-oncogene RET; RTK = receptor tyrosine kinase; TCR = T-cell Receptor; SUN = sunitinib; TEAE = Treatment Emergent Adverse Event; TKI = Tyrosine Kinase Inhibitor; VEGF = Vascular Endothelial Growth Factor; VEGFR = Vascular Endothelial Growth Factor Receptor.
References
- KEYTRUDA (pembrolizumab) Summary of Product Characteristics.
- KISPLYX (lenvatinib) Summary of Product Characteristics.
- Ciciola P et al. J Clin Med. 2020;9(3):675.
- Kudo M. Cancers (Basel). 2020;12(5):1089.
- Lee WS et al. Exp Mol Med. 2020;52(9):1475–1485.
- Rassy E et al. Ther Adv Med Oncol. 2020;12:1758835920907504.
- Pardoll DM. Nat Rev Cancer. 2012;12(4):252–264.
- Kudo M. Liver Cancer. 2018;7(1):1–19.
- Kantar Health. Treatment Architecture: Renal Cell Carcinoma. CancerMPact®. EU5. 2020;1–89.
- Motzer R et al. N Engl J Med 2021;384:1289–1300 (and supplementary materials).
- Choueiri TK et al. Lancet Oncol 2023;24;228–238.
- Choueiri TK et al. Presented at the Kidney Cancer Research Summit (KCRS) congress 2021, October 7–8. Oral presentation.
Supporting documentation
KEYTRUDA Prescribing Information for Great Britain & Northern Ireland
KISPLYX Prescribing information for Great Britain & Northern Ireland
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website
