Efficacy and Results
Efficacy and Results
LYFNUA®▼ (gefapixant)
Prescribing Information [External link]
LYFNUA is indicated in adults for the treatment of refractory and unexplained chronic cough1
LYFNUA is available by private prescription only and is not available via the NHS
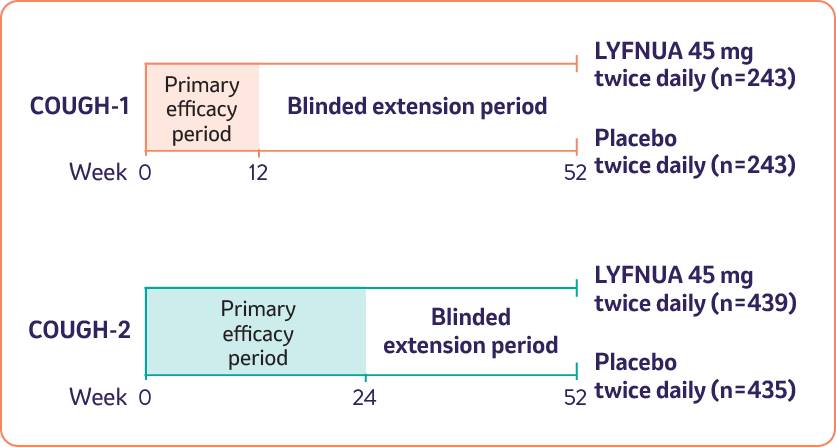
LYFNUA®▼ (gefapixant) was evaluated in two phase 3 clinical trials, including extensions up to week 521
-
Primary Endpoint: Reduction in 24-hour cough frequency relative to placebo
-
Secondary Endpoints: Reduction in awake cough frequency and cough-specific quality of life
-
Patients with a range of ages, cough duration and common conditions associated with refractory chronic cough were enrolled in both trials1,2
*Patients were randomised to twice-daily doses of LYFNUA 45 mg, 15 mg (not shown), or placebo.1
-
Safety was evaluated in 1369 patients from COUGH-1 and COUGH-2 treated with LYFNUA (15 mg or 45 mg twice daily) over 52 weeks1
*A LYFNUA dose of 15MG was also included within the trial however this did not demonstrate a statistically significant reduction in 24-hour cough frequency in either the COUGH-1 or COUGH-2 study
LYFNUA helps reduce 24-hour cough frequency and improve cough-specific quality of life1
Patients taking LYFNUA 45 mg TWICE DAILY showed a statistically significant reduction in 24-hour cough frequency relative to placebo1
COUGH-1: Reduction in 24-hour cough frequency at 12 weeks versus placebo was -18.52%* (95% CI: -32.76, -1.28; p=0.036).
Disclaimer: An absolute risk reduction has not been included as it is a metric for an event-based variable (not a change from baseline variable).
COUGH-1 Primary Endpoint: 24 hour cough frequency over time
Adapted from Figure 1 and Table 2 in SmPC
Based on a population pharmacokinetic analysis, age, body weight, gender, ethnicity, and race do not have a clinically meaningful effect on the pharmacokinetics of LYFNUA.1
*Missing baseline values were imputed based on gender and region, followed by multiple imputation of the missing data (m=50 imputed datasets) for all follow-up visits using treatment, gender, region, and the other follow-up visits as covariates. Following imputation, an analysis of covariance (ANCOVA) model was conducted at the time point of interest, adjusting for covariates of treatment, baseline, gender, and region.
Patients taking LYFNUA 45 mg TWICE DAILY showed a statistically significant reduction in 24-hour cough frequency relative to placebo1
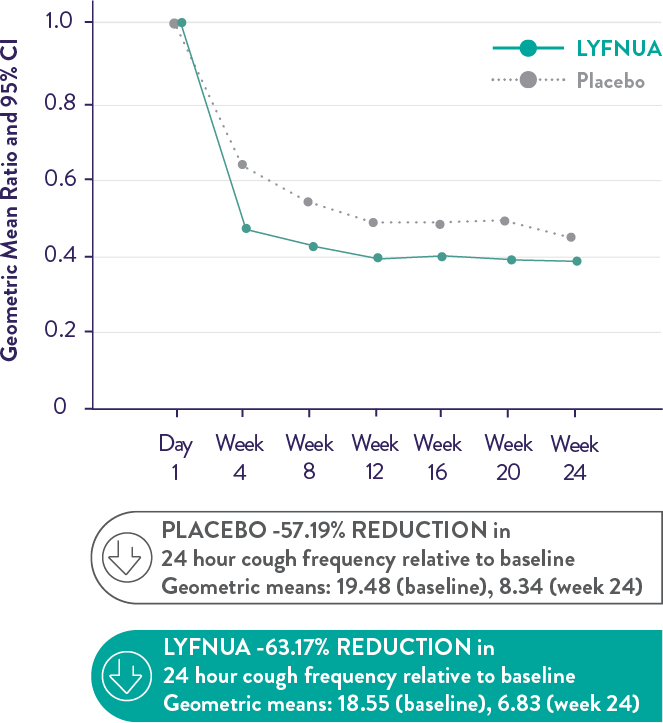
COUGH-2: Reduction in 24-hour cough frequency at 24 weeks versus placebo was -13.29%* (95% CI: -24.74, -0.10; p=0.048).
Disclaimer: An absolute risk reduction has not been included as it is a metric for an event-based variable (not a change from baseline variable).
COUGH-2 Primary Endpoint: 24 hour cough frequency over time
Adapted from Figure 1 and Table 2 in SmPC
Based on a population pharmacokinetic analysis, age, body weight, gender, ethnicity, and race do not have a clinically meaningful effect on the pharmacokinetics of LYFNUA.1
*Missing baseline values were imputed based on gender and region, followed by multiple imputation of the missing data (m=50 imputed datasets) for all follow-up visits using treatment, gender, region, and the other follow-up visits as covariates. Following imputation, an analysis of covariance (ANCOVA) model was conducted at the time point of interest, adjusting for covariates of treatment, baseline, gender, and region.
COUGH-2: Cough-specific Quality of Life
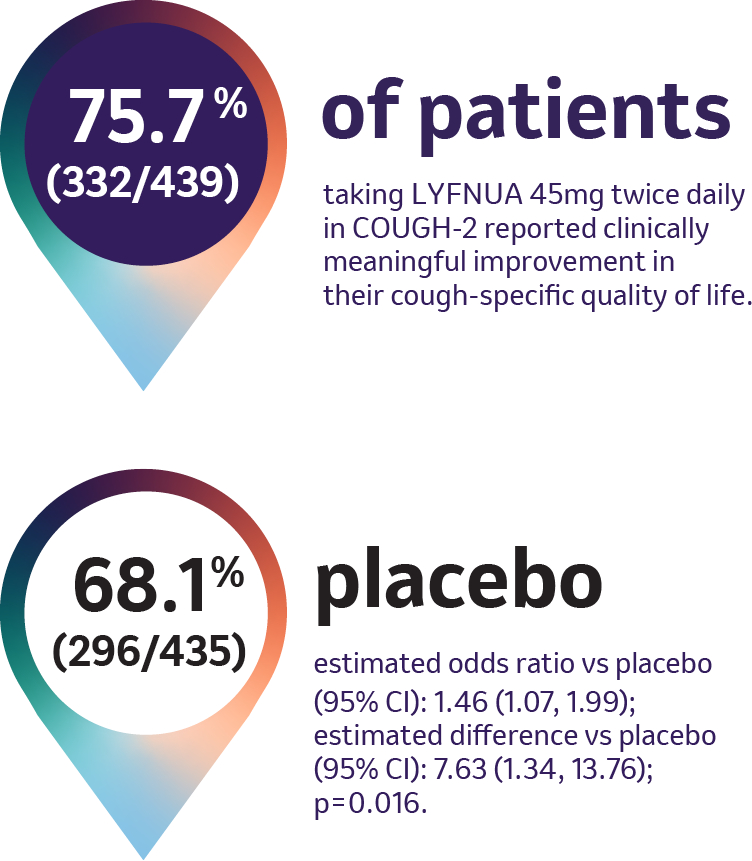
Adapted from Table 3 in SmPC
COUGH-2 was specifically designed to assess the impact of Lyfnua on cough-specific quality of life relative to placebo as measured by the Leicester Cough Questionnaire (LCQ) (possible score ranges from 3 to 21, with higher scores indicating a better quality of life). The Leicester Cough Questionnaire is a validated, multidimensional, patient-reported, health related quality of life questionnaire commonly used in clinical studies assessing cough. It evaluates physical, social, and psychological components of cough-specific quality of life. A ≥1.3 point increase from baseline in the LCQ total score was defined as clinically meaningful.1 Subjective measures of cough-specific quality of life should be interpreted in combination with objective measures. Subjective measures of cough-specific quality of life may be impacted by a relative lack of specificity for cough, potentially also capturing off-target changes in health due to other potential effects of antitussives.3
Always refer to the full Summary of Product Characteristics before prescribing for up to-date and complete safety considerations to help minimise the risks associated with the use of LYFNUA.
Please click here for the Summary of Product Characteristics [External link]
Stay informed about the latest on LYFNUA
Click the sign up button and consent to receive promotional emails, resources and invitations to meetings
References
-
LYFNUA Summary of Product Characteristics.
-
Muccino DR et al. ERJ Open Res. 2020;6:00284-2020.
-
Turner, RF, Birring, SS. Journal of Thoracic Disease. 2023;15(4):2288-22.
Supporting documentation
LYFNUA®▼ (gefapixant)
Prescribing Information (Great Britain)
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website