Safety and Tolerability
Safety and Tolerability
LYFNUA®▼ (gefapixant)
Prescribing Information [External link]
LYFNUA is indicated in adults for the treatment of refractory and unexplained chronic cough1
LYFNUA is available by private prescription only and is not available via the NHS
LYFNUA®▼ (gefapixant) is non-narcotic with a safety profile established in two 52-week phase 3 clinical trials1,2
Adverse reactions in patients treated with LYFNUA in two phase 3 clinical studies (COUGH-1 AND COUGH-2)3
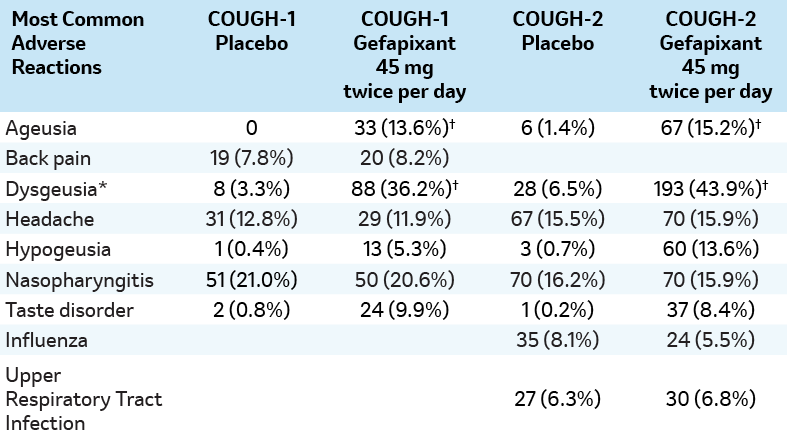
Data are n or n (%). †p≤0.001. *Dysgeusia was commonly reported as taste bitter, taste metallic or taste salty.
Note: Ageusia was defined as a loss of taste. Hypogeusia was defined as diminished taste.3
- Adverse reactions resulting in discontinuation occurred in 22% of patients receiving LYFNUA.1
- The most frequently reported adverse reactions leading to discontinuation of LYFNUA were dysgeusia (9%) and ageusia (4%).1
Dosing
One 45 mg pill taken orally twice daily with or without food1

Instruct patients that if they miss a dose, they should skip that dose and take the next dose at the regular scheduled time.
Patients should not double their next dose or take more than the prescribed one.1
Please refer to the Summary of Product Characteristics for guidance on any dosage adjustments.
Special populations1

*eGFR, estimated glomerular filtration rate.
Frequencies are defined as very common (≥ 1/10), common (≥ 1/100 to <1/10), uncommon (≥ 1/1,000 to <1/100), rare (≥ 1/10,000 to <1/1,000), and very rare (<1/10,000).
Adverse reactions in patients treated with LYFNUA in two phase 3 clinical studies (COUGH-1 AND COUGH-2)1
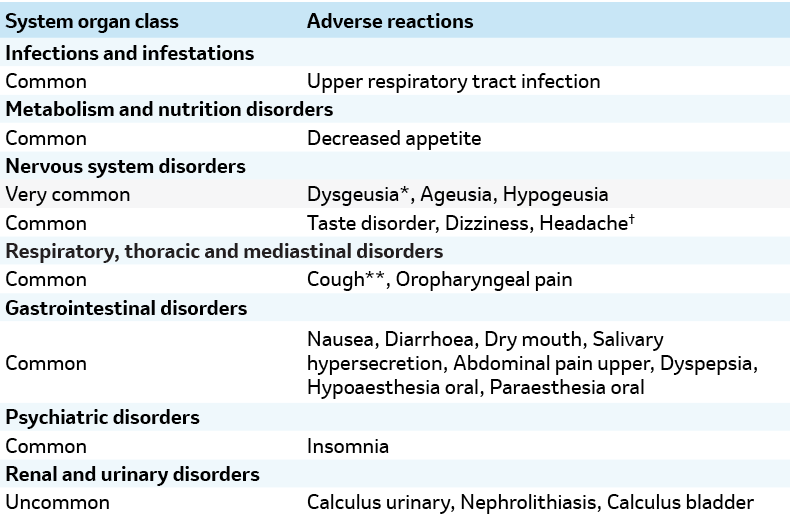
*Dysgeusia was commonly reported as taste bitter, taste metallic or taste salty.
† Headache was reported in a phase 3b clinical study in female patients with cough-induced stress urinary incontinence (C-SUI).
** Cough includes reports of ‘worsening’, ‘exacerbation’, ‘increase’, or ‘increased’ cough. Note: Ageusia was defined as a loss of taste. Hypogeusia was defined as diminished taste.3
- Adverse reactions resulting in discontinuation occurred in 22% of patients receiving LYFNUA.1
- The most frequently reported adverse reactions leading to discontinuation of LYFNUA were dysgeusia (9%) and ageusia (4%).1
Dosing
One 45 mg pill taken orally twice daily with or without food1

Instruct patients that if they miss a dose, they should skip that dose and take the next dose at the regular scheduled time.
Patients should not double their next dose or take more than the prescribed one.1
Please refer to the Summary of Product Characteristics for guidance on any dosage adjustments.
Special populations1

*eGFR, estimated glomerular filtration rate.
Always refer to the full Summary of Product Characteristics before prescribing for up to-date and complete safety considerations to help minimise the risks associated with the use of LYFNUA.
Please click here for the Summary of Product Characteristics [External link]
Stay informed about the latest on LYFNUA
Click the sign up button and consent to receive promotional emails, resources and invitations to meetings
References
- LYFNUA Summary of Product Characteristics.
- Muccino DR, Green S. Update on the clinical development of gefapixant, a P2X3 antagonist for the treatment of refractory chronic cough. Pulm Pharmacol Ther. 2019;56:75-78.
- McGarvey L, Gibson PG. What is Chronic Cough? Terminology. J Allergy Clin Pract. 2019;7:1711-1714.
Supporting documentation
LYFNUA®▼ (gefapixant)
Prescribing Information (Great Britain)
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website