About PREVYMIS
About PREVYMIS®▼ (letermovir)
PREVYMIS® Infusion Prescribing Information Great Britain / Northern Ireland [External links]
PREVYMIS® Tablets Prescribing Information Great Britain / Northern Ireland [External links]
A prophylactic option for CMV reactivation in adult R+ allogeneic HSCT recipients
PREVYMIS is the first and only CMV terminase inhibitor indicated for prophylaxis of cytomegalovirus (CMV) reactivation and disease in adult CMV-seropositive recipients [R+] of an allogeneic haematopoietic stem cell transplant (HSCT).1
With its novel mechanism of action, PREVMYIS targets a component of the terminase complex involved in viral DNA cleavage and packaging.1 Cross-resistance is therefore not likely with agents with a different mechanism of action.1

Oral and IV administration
PREVYMIS is now available as either tablets or intravenous (IV) infusion1,2
The flexible administration options mean that cytomegalovirus (CMV) prophylaxis can be initiated early, from Day 0 post-haematopoietic stem cell transplant through to Day 100,1,2 without disruption if a patient becomes unable to take oral therapy.3
How is PREVYMIS taken?1,2
The recommended dose of PREVYMIS is 480 mg once daily. If PREVYMIS (oral or IV formulation) is co-administered with ciclosporin, the dosage should be decreased to 240 mg once daily.
240 mg film-coated TABLETS  | 240 mg concentrate for solution for INFUSION  |
|---|---|
| For oral use only | For IV use only |
| Each film-coated tablet contains 240 mg of letermovir | Each vial contains 240 mg (12 mL per vial) of letermovir |
| Tablet(s) should be swallowed whole | Requires dilution prior to administration through a sterile 0.2 micron or 0.22 micron polyethersulfone (PES) in-line filter |
| Can be taken with or without food | Should be administered by IV infusion via peripheral or central venous catheter using a total time of approximately 60 minutes. The entire contents of the IV bag should be administered |
| Should not be divided, crushed or chewed | Should not be administered as an IV push or bolus |
Images are for illustrative purposes only
Switching between formulations1–4
- Tablets and concentrate for solution for infusion can be used interchangeably without dose adjustment at the discretion of the prescriber
- PREVYMIS should be taken at the same time each day; the formulation can be switched at the next planned dose

CMV is associated with an increased risk of overall mortality in the first year post-HSCT, independent of pre-emptive therapy use5

In a Phase 3 clinical trial, PREVYMIS demonstrated:
- Superior efficacy in CMV prophylaxis at Week 24 compared to placebo1
- Lower all-cause mortality vs. placebo at Week 24 post-transplant6*
- A generally well-tolerated safety profile1
- Once-daily oral dosing – as early as Day 0 and through Day 100 post-transplant1
Start PREVYMIS as early as Day 0 post-transplant in adult CMV-seropositive (R+) allogeneic HSCT patients1
(Not an actual patient.) *Based on a pre-specified exploratory endpoint
Study overview
Study design
CMV prophylaxis was assessed in a randomised, multi-centre, double-blind, placebo-controlled, pivotal, Phase 3 study of adult CMV-seropositive recipients (R+) of an allogeneic HSCT (haematopoietic stem cell transplant).* Subjects were randomised (2:1) to receive either PREVYMIS or placebo and stratified by investigational site and risk (high vs. low) for CMV reactivation at the time of study entry (n=565).1
Median time to randomisation was 9 days after transplantation.1
*Full analysis set included randomised subjects who received at least 1 dose of study medicine and excluded subjects with detectable CMV DNA at baseline.1
Additional study design details
- The protocol suggested thresholds for the initiation of pre-emptive therapy were >150 copies/mL for high-risk patients and
>300 copies/mL for low-risk patients through Week 14 post-transplant and a threshold of >300 copies/mL for all patients thereafter6 - Patients were considered high risk if they met 1 or more of the following criteria:1
- Human leukocyte antigen (HLA)-related (sibling) donor with at least 1 mismatch at 1 of the following 3 HLA-gene loci: HLA-A, -B, or -DR
- Haploidentical donor
- Unrelated donor with at least 1 mismatch at 1 of the following 4 HLA-gene loci: HLA-A, -B, -C, or -DRB1
- Use of umbilical cord blood as the stem cell source
- Use of ex vivo T cell–depleted grafts
- Grade 2 or greater GVHD, requiring systemic corticosteroids
Efficacy endpoints
The primary endpoint was defined as the proportion of patients with clinically significant CMV infection at Week 24.1
For the primary endpoint, patients were also considered failures of CMV prophylaxis if they:
- Discontinued from the study prior to Week 24 post-transplant
- Were missing an outcome at Week 24 post-transplant
Results
PREVYMIS demonstrated superior efficacy over placebo in the primary end point: the proportion of patients with clinically significant CMV infection at Week 24.1*
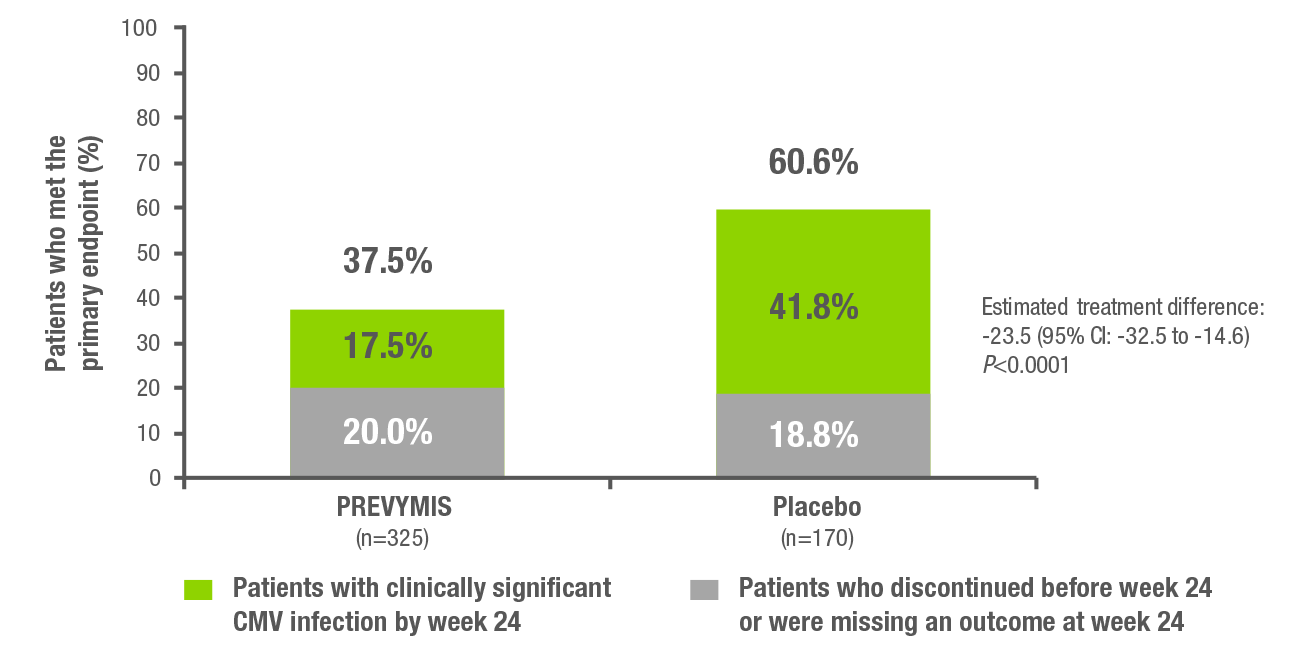
Adapted from PREVYMIS Summary of Product Characteristics1
*Clinically significant CMV infection was defined as the incidence of CMV viraemia warranting anti-CMV pre-emptive therapy or the occurrence of CMV end-organ disease.1
- The treatment difference was driven by clinically significant CMV infection, with otherwise similar proportions of patients who discontinued the trial or with missing outcomes1
Adverse events
PREVYMIS demonstrated a generally well-tolerated safety profile for adult allogeneic HSCT (haematopoietic stem cell transplant) CMV (cytomegalovirus) prophylaxis.1
Overall incidence of adverse events (AE) was comparable to that in the placebo group.1
AEs reported in ≥1% of patients and at a frequency greater than placebo.7
| AE | PREVYMIS (n=373) | Placebo (n=192) |
|---|---|---|
| Nausea | 7.2% | 3.6% |
| Diarrhoea | 2.4% | 1.0% |
| Vomiting | 1.9% | 1.0% |
Additional safety information
Contraindications1,2
- Hypersensitivity to the active substance or to any of the excipients
- Concomitant administration with pimozide
- Concomitant administration with ergot alkaloids
- Concomitant administration with St. John’s wort (Hypericum perforatum)
- When letermovir is combined with ciclosporin: concomitant use of dabigatran, atorvastatin, simvastatin, rosuvastatin or pitavastatin is contraindicated
References
- PREVYMIS 240 mg film-coated tablets Summary of Product Characteristics.
- PREVYMIS 240 mg concentrate for solution for infusion Summary of Product Characteristics.
- Marty FM et al. Letermovir prophylaxis for cytomegalovirus in haematopoietic-cell transplantation. N Engl J Med. 2017;377:2433–2444. Supplementary Appendix.
- PREVYMIS Patient Information Leaflet.
- Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haematopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3:e119–e127.
- Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377:2433–2444.
- Data on file; AINF-1251990-0000, available upon request from MSD.
Supporting documentation
PREVYMIS® Infusion Prescribing Information Great Britain / Northern Ireland
PREVYMIS® Tablets Prescribing Information Great Britain / Northern Ireland
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website
