ADEMPAS® (riociguat)
About ADEMPAS® (riociguat)
Prescribing Information (United Kingdom) [External link]
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website
Indication
ADEMPAS is a member of a class of therapeutic agents called ‘soluble guanylate cyclase stimulators (sGC stimulators)’, and is used for the treatment of pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH).1
In PAH, ADEMPAS is licenced as monotherapy or in combination with endothelin receptor antagonists (ERAs), for the treatment of adult patients with PAH with WHO functional class (FC) II to III, in order to improve exercise capacity.1
In paediatric patients aged less than 18 years of age and body weight ≥50 kg, ADEMPAS is licensed for the treatment of PAH in combination with ERAs for patients in WHO FC II to III.1
In CTEPH, ADEMPAS is licenced for the treatment of adult patients with WHO functional class II to III with:1
- inoperable CTEPH
- persistent or recurrent CTEPH after surgical treatment
to improve exercise capacity.
Healthcare Professionals are to consult the SmPC for further information to minimise the risks associated with the use of the medicine before making any prescribing decisions.
Mode of action
ADEMPAS has a dual mode of action. It increases cGMP levels by both sensitising sGC to endogenous NO and directly stimulating sGC independently of NO.1,2
These images were created for MSD.
NO = Nitric Oxide; sGC = soluble Guanylate Cyclase; cGMP = cyclic Guanosine Monophosphate; GTP = Guanosine Triphosphate.
Why is this important?
- Intracellular cGMP has an important role in regulating processes that influence vascular tone, proliferation, fibrosis and inflammation1
- Pulmonary hypertension is associated with endothelial dysfunction, impaired synthesis of NO, and insufficient stimulation of the NO-sGC-cGMP pathway1
- ADEMPAS aims to restore the NO-sGC-cGMP pathway, resulting in an improvement of pulmonary vascular hemodynamics and an increase in exercise ability1
ADEMPAS is the only oral pharmacological therapy licensed for inoperable CTEPH or persistent or recurrent CTEPH after surgical treatment.1,4
CHEST-2 study overview – CTEPH (open-label extension)
Study design
237 patients entered CHEST-2.5 CHEST-2 was a multicentre, open-label, single-group study conducted at 71 out of the 89 centres (across 25 out of the 26 countries) that participated in CHEST-1.7
CHEST-1 was a randomised, double-blind, multi-national, placebo controlled, phase III study conducted in 261 adult patients with inoperable chronic thromboembolic pulmonary hypertension (CTEPH) (72%) or persistent or recurrent CTEPH after pulmonary endarterectomy (PEA; 28%). During the first 8 weeks riociguat was titrated every 2-weeks based on the patient’s systolic blood pressure and signs or symptoms of hypotension to the optimal individual dose (range 0.5 mg to 2.5 mg 3 times daily) which was then maintained for a further 8 weeks. The primary endpoint of the study was the placebo adjusted change from baseline in 6-minute walk distance (6MWD) at the last visit (week 16). At the last visit, the increase in 6MWD in patients treated with riociguat was 46 m (95% confidence interval (CI): 25 m to 67 m; p<0.0001), compared to placebo.5
CHEST-2 was a multicentre, open-label, single-group study conducted at 71 out of the 89 centres (across 25 out of the 26 countries) that participated in CHEST-1. Patients in the former riociguat group started CHEST-2 at the riociguat dose they received at the end of CHEST-1, while former placebo patients started CHEST-2 at 1 mg three times daily. During the 8-week double-blind dose-adjustment phase of CHEST-2, patients in the former placebo group were individually adjusted up to a maximum of 2.5 mg three times daily according to systolic blood pressure (SBP) and symptoms of hypotension, and patients in the former riociguat group continued on the dose they were receiving at the end of CHEST-1 while receiving sham titration. During the open-label study phase, investigators could adjust the riociguat dose (up to a maximum dose of 2.5 mg three times daily) according to the patient’s need, considering SBP, side-effects and progression of underlying CTEPH.5
Outcome measures
- Primary outcome: safety and tolerability5
-
Exploratory endpoints:
Change from baseline in 6MWD, NT-proBNP (N-terminal pro-brain natriuretic peptide) concentration, WHO FC, Borg dyspnoea score, quality of life, overall survival, and clinical worsening-free survival5
Adverse events/safety information
Safety results of ADEMPAS in CHEST-2 were consistent with previously reported findings from CHEST-1. The incidence of AEs during CHEST-2 was generally similar in patients with inoperable CTEPH and those with persistent or recurrent pulmonary hypertension after PEA. The most common serious AEs were syncope (23 [10%] of 237), worsening pulmonary hypertension (18 [8%] ), and right ventricular failure (16 [7%]).5,7
There were 13 deaths during CHEST-2, none of which were considered to be study drug-related by the investigators.7
14 (6%) of 237 patients discontinued riociguat therapy because of AEs.5
Results
Please note: These are exploratory endpoints and therefore the results should be interpreted with caution.
The results at 2 years from the CHEST-2 trial showed that long-term use of ADEMPAS is generally well-tolerated in patients with CTEPH.5
Building on the results of CHEST-1 the 2-year data from CHEST-2 further showed that treatment with ADEMPAS leads to sustained improvement in 6MWD and WHO FC in patients with inoperable CTEPH and persistent/recurrent PH after PEA compared to baseline.5
At 2 years, overall survival was 93% (95% Cl 89-96) and clinical worsening-free survival was 82% (77-87).5
Improvements in 6MWD and WHO FC observed in CHEST-1 persisted for up to 1 year in CHEST-2. In the observed population at 1 year, mean±SD 6MWD had changed by +51±62 m (n=172) versus CHEST-1 baseline (n=237), and WHO FC had improved/stabilised/worsened in 47/50/3% of patients (n=176) versus CHEST-1 baseline (n=236).7
In patients who received placebo during CHEST-1, although the incidence of clinical worsening events was similar compared with former riociguat patients, improvements in 6MWD and WHO FC after transitioning to riociguat treatment did not fully catch up to the former riociguat group by the 1-year cut-off. This potentially highlights the importance of initiating the appropriate treatment as early as possible.5,7
ADEMPAS provides a potential treatment option for stable patients receiving a PDE5i who are not achieving low-risk.8
REPLACE study overview – PAH
REPLACE is the first head to head study demonstrating greater clinical improvement for patients with PAH switching to ADEMPAS versus continuing with PDE5i8
Clinical improvement was defined as an absence of clinical worsening and prespecified improvements in at least two of three variables (6MWD, WHO functional class, and N-terminal prohormone of brain natriuretic peptide) by week 24.
Clinical worsening was defined as death from any cause, hospitalisation for worsening PAH (non-elective hospitalisation due to PAH or initation of parenteral prostanoid therapy), or disease progression (decrease in 6MWD more than or equal to 15% on two separate days plus either worsening WHO functional class, need for new PAH-targeted medication, or decompensated right-sided heart failure).
Study design
REPLACE was a clinical study in 224 adult patients with PAH at intermediate risk of 1-year mortality (based on European Society for Cardiology-European Respiratory Society guideline thresholds for 6MWD and WHO functional class). The study consisted of a 14-day screening period, 24 weeks of randomised treatment, and a 30-day safety follow-up period.8,a
This was a prospective, open-label study in which patients were randomly assigned (1:1) to remain on PDE5i treatment (oral sildenafil (≥60 mg per day) or oral tadalafil (20-40 mg per day) or switch to ADEMPAS (up to 2.5 mg three times a day)8. Patients taking combination therapy with an endothelin receptor antagonist at baseline continued, regardless of study randomisation.
It was powered to evaluate the efficacy of ADEMPAS in adult patients with symptomatic PAH at intermediate risk who were receiving a stable dose of a PDE5i with or without an ERA and not at treatment goal8,a
Efficacy endpoints
The primary endpoint was a composite endpoint of clinical improvement from baseline to week 24, (in the absence of clinical worsening) in at least two of the following criteria:8,a,9,c
Prespecified improvements in at least 2 out of 3 parameters:
- Improvement in 6MWD of ≥10% or ≥30 m
- Improvement to WHO FC I or II
- Decrease in NT-proBNP of ≥30%
Secondary endpoints8,a,d
Change from baseline to Week 24 in;
- 6MWD
- NT-proBNP
- WHO FC
Time to first clinical worsening event
Exploratory endpoints8,a
Risk assessment at baseline, 16 weeks, and 24 weeks using 3 risk score calculators:
- REVEAL
- COMPERA
- French Pulmonary Hypertension Network non-invasive
aIntermediate risk determination based on WHO FC and 6MWD.
bRequired washout period of 24 hours after last dose of sildenafil and 48 hours after last dose of tadalafil.
cClinical worsening defined as death from any cause; hospitalization for worsening PAH (non-elective hospitalization due to PAH or initiation of parenteral prostanoid therapy); or disease progression (decrease in 6MWD ≥15% on two separate days plus either worsening WHO FC, need for new PAH-targeted medication, or decompensated right-sided heart failure).
dSecondary endpoints were tested hierarchically in the following order: 6MWD, NT-proBNP, WHO FC, and clinical worsening.
Results
In patients on a PDE5i and at intermediate riska, transitioning to ADEMPAS helped more PAH patients achieve clinical improvement compared to continuing treatment with a PDE5i8,b
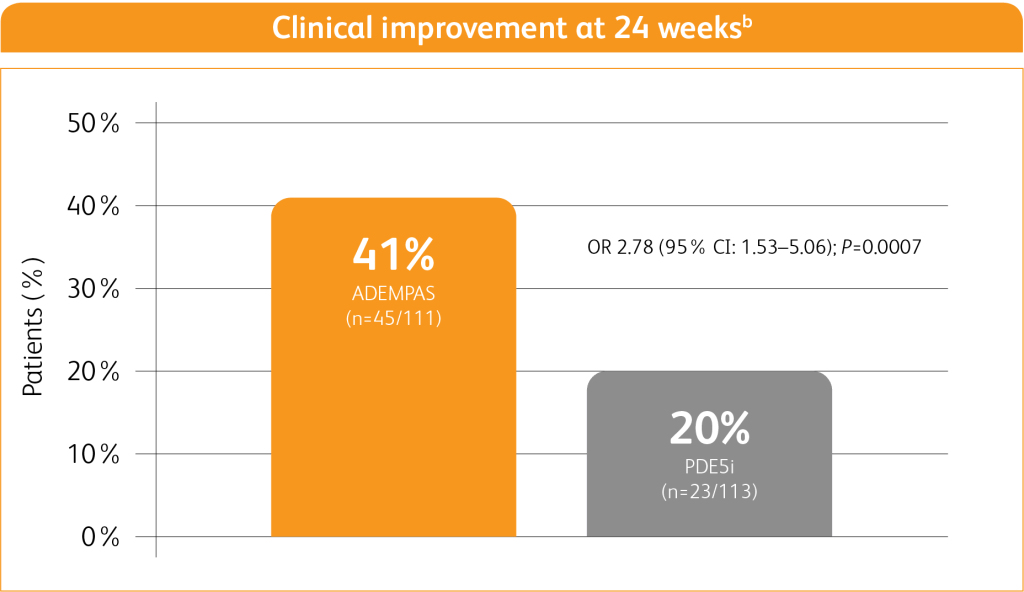
Adapted from Hoeper M M, Al-Hiti H et al. 20218
1% of patients receiving ADEMPAS experienced a clinical worsening event vs 9% of patients who continued PDE5i treatment8,c
aIntermediate risk determined based on WHO FC and 6MWD.
bClinical improvement at 24 weeks defined as prespecified improvements in at least 2 out of 3 parameters (6MWD, WHO FC, and/or NT-proBNP) and absence of clinical worsening.
cPatients who experienced clinical worsening are a subgroup of those who did not achieve the endpoint. Clinical worsening defined as death from any cause; hospitalization for worsening PAH (non-elective hospitalization due to PAH or initiation of parenteral prostanoid therapy); or disease progression (decrease in 6MWD ≥15% on 2 separate.
Adverse events
The safety results from patients switching to ADEMPAS were consistent with the known safety profile of ADEMPAS.8
ADEMPAS was generally well-tolerated by patients who switched from a PDE5i:8
- Overall adverse event (AE) rates were similar between treatment groups (71% [79/111] in the ADEMPAS group vs. 66% [75/114] in the PDE5i group), with a higher incidence of serious AEs in the PDE5i group than in the ADEMPAS group (17% vs. 7%, respectively)
- The most frequently reported AEs with ADEMPAS were hypotension (14% [15/111], headache (13% [14/111]), and dyspepsia (9% [10/111]), headache and dyspepsia
- The most frequently reported AEs with PDE5is were headache (7% [8/114]), cough (6% [7/114], and upper respiratory tract infection (6% [7/114]), cough and upper respiratory tract infections
Refer to the Summary of Product Characteristics for full information on adverse reactions.

Adapted from Hoeper M M, Al-Hiti H et al. 20218
If you are initiating an eligible patient on ADEMPAS, we have the home monitoring pack service available to support. The home monitoring pack has been designed to help your patients as they start their treatment on ADEMPAS. This consists of a blood pressure monitor and associated items to help your patients monitor their blood pressure during the titration period.
The loan of the Blood Pressure equipment is supported by MSD UK Ltd. To order a home monitoring pack for your centre, please contact our third party supplier Uniphar Commercial (E4H) UK Limited using the following contact details:
Phone: 0800 012 6055
Email: hmp@hcpconnect-uniphar.com
Additional safety information1
Contraindications
- Co-administration with PDE5 inhibitors (such as sildenafil, tadalafil, vardenafil)
- Severe hepatic impairment (Child-Pugh C)
- Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of the ADEMPAS SmPC
- Pregnancy
- Co-administration with nitrates or nitric oxide donors (such as amyl nitrite) in any form including recreational drugs called ‘poppers’
- Patients ≥12 years with systolic blood pressure <95 mmHg at treatment initiation
- Children aged 6 to <12 years with systolic blood pressure <90 mmHg at treatment initiation
- Patients with pulmonary hypertension associated with idiopathic interstitial pneumonias (PH-IIP)
- Concomitant use with other soluble guanylate cyclase stimulators
Side effects
In adults
Very common: dizziness, headache, dyspepsia, diarrhoea, nausea, vomiting, peripheral oedema.
Common: gastroenteritis, anaemia, palpitations, hypotension, haemoptysis, epistaxis, nasal congestion, gastritis, gastro-oesophageal reflux disease, dysphagia, gastrointestinal and abdominal pains, constipation, abdominal distension.
Uncommon: pulmonary haemorrhage (incl. cases with fatal outcome).
In children
Most common adverse reactions including the long-term extension phase were hypotension and headache.
Overall, the safety data is consistent with the safety profile observed in adults.
References
- ADEMPAS Summary of Product Characteristics.
- O’Callaghan DS, Savale L, Montani D, et al. Treatment of pulmonary arterial hypertension with targeted therapies. Nature Rev Drug Cardiol. 2011;8:526–538.
- Stasch J-P, Pacher P, Evgenov OV, et al. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123:2263–2273.
- Wilkens, H et al. Chronic thromboembolic pulmonary hypertension (CTEPH) Updated Recommendations from the Cologne Consensus Conference 2018. Int J Cardiol. 2018;1;272S:69-78.
- Simonneau G, D’Armini AM, Ghofrani H-A, et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;10.1016/S2213-2600(16)30022–4.
- Simonneau G, D’Armini AM, Ghofrani H-A, et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Supplementary appendix. Lancet Respir Med. 2016;10.1016/S2213-2600(16)30022–4.
- Simonneau G, D’Armini A, Ghofrani H, et al 2015. Riociguat For The Treatment Of Chronic Thromboembolic Pulmonary Hypertension: A Long-Term Extension Study (CHEST-2). [online] Available at: https://erj.ersjournals.com/content/45/5/1293.long.
- Hoeper M M, Al-Hiti H et al. Switching to riociguat versus maintenance therapy with phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension (REPLACE): a multicentre, open-label, randomised controlled trial. Lancet Respir Med. 2021;9(6):573-584.
Supporting documentation
Prescribing Information (United Kingdom)
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website
