Adjuvant KEYTRUDA® (pembrolizumab) in patients with early-stage renal cell carcinoma
Adjuvant KEYTRUDA® (pembrolizumab) in patients with early-stage renal cell carcinoma
Prescribing Information [External link]
The first adjuvant treatment option for patients with RCC at increased risk of recurrence post-nephrectomy1
For use in

KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with renal cell carcinoma at increased risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions.1
Clinical trial

KEYNOTE-564 was a Phase III landmark, multicentre, randomised, double-blind, placebo-controlled clinical study, evaluating the efficacy and safety of KEYTRUDA monotherapy given as adjuvant treatment in RCC patients at intermediate–high or high risk of recurrence, or M1 NED (N=994).2
For further information about KEYNOTE-564, click the above image.
Recommended for desktop viewing.
Eligible patients

KEYTRUDA is a suitable option for:
Intermediate–high risk patients2
- pT2 with Grade 4 or sarcomatoid, N0, M0
- pT3, any grade, N0, M0
High-risk patients2
- pT4, any grade, N0, M0 any pT, any grade, N+, M0
M1 NED patients2
- M1 NED after resection of oligometastatic sites ≤1 year after nephrectomy
The 2024 ESMO guidelines recommend considering KEYTRUDA in the adjuvant setting3
The latest ESMO guidelines state that the TNM prognostic classification used in KEYNOTE-564 is clinically relevant and is now the preferred risk classification for operable disease.3

Adjuvant KEYTRUDA should be considered for patients with intermediate–high or high-risk operable clear cell RCC (as defined by the KEYNOTE-564 criteria) after careful patient counselling regarding potential long-term adverse events.3 Treatment should start within 12 weeks of surgery and continue for up to 1 year.3

Adjuvant KEYTRUDA can be offered to patients with oligometastatic disease who have undergone complete resection (M1 and NED).3

Classification of ESMO recommendations
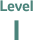
“Evidence from at least one large randomised, controlled trial of good methodological quality (low potential for bias) or meta-analyses of well-conducted randomised trials without heterogeneity.”4
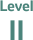
“Small randomised trials or large randomised trials with a suspicion of bias (lower methodological quality) or meta-analyses of such trials or of trials with demonstrated heterogeneity.”4

“Strong evidence for efficacy with a substantial clinical benefit, strongly recommended.”4

“Strong or moderate evidence for efficacy but with a limited clinical benefit; generally recommended.”4

“The highest grades of the ESMO-MCBS in the curative setting are A and B, which indicate a substantial magnitude of clinical benefit.”5
Dosing schedule

The recommended dose of KEYTRUDA in adults is an intravenous infusion over 30 minutes of either:1
200 mg every 3 weeks or 400 mg every 6 weeks
Duration of treatment

Patients should be treated with KEYTRUDA:1
- until disease progression, or
- until unacceptable toxicity, or
- for 1 year
Connect with us
To share your thoughts on these data and arrange a meeting at your convenience, email us at: msdukoncology@msd.com
Refer to the Summary of Product Characteristics and Risk Minimisation Materials available on the emc website before prescribing, in order to help reduce the risk associated with KEYTRUDA.
Other useful resources
Training and resources
Abbreviations
ESMO, European Society for Medical Oncology; M0, without distant metastases; M1, with distant metastases; MCBS, Magnitude of Clinical Benefit Scale; N+, involvement of nearby nodes; N0, without nodal involvement; NED, no evidence of disease; pT, pathological primary tumour; RCC, renal cell carcinoma; TNM, Tumour, Node, Metastasis.
References
- KEYTRUDA® Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/2498. Accessed: February 2025.
- Choueiri TK, et al. N Engl J Med 2021;385:683–694.
- Powles T, et al. Ann Oncol 2024;S0923-7534(24)00676-8.
- ESMO. Standard Operating Procedures for ESMO Clinical Practice Guidelines v2.3. Available at: https://www.esmo.org/content/download/77789/1426712/1/ESMO-Clinical-Practice-Guidelines-Standard-Operating-Procedures.pdf. Accessed: August 2024.
- ESMO. ESMO Magnitude of Clinical Benefit Scale v1.1. Available at: https://www.esmo.org/content/download/288502/5736211/1/esmo-mcbs-booklet.pdf. Accessed: August 2024.
Supporting documentation
Prescribing Information [External link]
By clicking the link above you will leave the MSD Connect website and be taken to the EMC PI portal website.

