About KEYTRUDA in triple-negative breast cancer (TNBC)

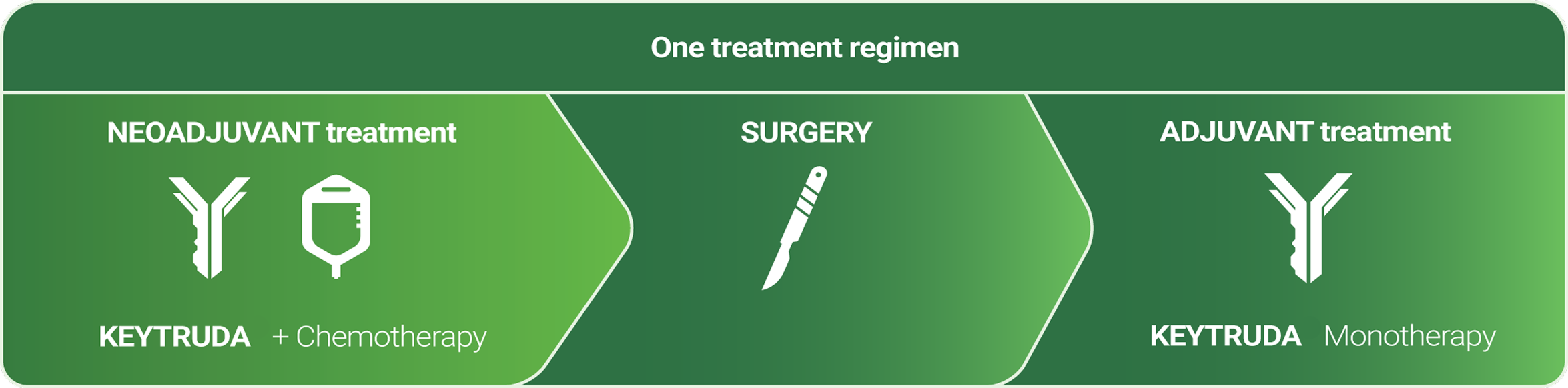
Neoadjuvant KEYTRUDA® + chemotherapy followed by adjuvant KEYTRUDA® monotherapy in patients with high-risk early-stage triple-negative breast cancer (TNBC)
Prescribing Information [External link]

The first and only immunotherapy indicated for early-stage TNBC as a neoadjuvant combination treatment followed by adjuvant monotherapy treatment.2
Hear from Professor Peter Schmid to discover the latest KEYNOTE-522 clinical trial data:
Click ‘CHAPTERS’ in the top-right corner of the video to easily navigate through the chapters below.
Prescribing Information [External link]
01:03: KEYTRUDA: Mechanism of action
01:26: KEYNOTE-522: Study design
02:05: KEYNOTE-522: Primary analyses
03:23: KEYNOTE-522: Exploratory analysis at 60-month follow-up
06:38: KEYNOTE-522: Exploratory analysis at 75-month follow-up
08:26: Summary
Prof. Peter Schmid, FRCP, MD, PhD
Professor Schmid is the clinical director of the Breast Cancer Centre and an honorary consultant medical oncologist at St. Bartholomew’s Hospital, London. He is also the Chair in Cancer Medicine and the Lead of the Centre of Experimental Cancer Medicine at Barts Cancer Institute, Queen Mary University London. Professor Schmid is the lead investigator of the KEYNOTE-522 trial in early-stage TNBC.
FRCP: Fellowship of Royal College of Physicians
Other useful resources
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE (NICE) GUIDANCE3
KEYTRUDA is recommended, as an option, with chemotherapy for neoadjuvant treatment and then continued alone as adjuvant treatment after surgery for adults with locally advanced, or early-stage triple- negative breast cancer (TNBC) at a high risk of recurrence.3
SCOTTISH MEDICINES CONSORTIUM (SMC) GUIDANCE4
KEYTRUDA is accepted for use in combination with chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment after surgery, for the treatment of adults with locally advanced, or early-stage TNBC at high risk of recurrence.4
TNBC: triple-negative breast cancer.
Refer to the Summary of Product Characteristics and Risk Minimisation Materials available on the emc website before prescribing, in order to help reduce the risk associated with KEYTRUDA.
References:
- Schmid P, Cortes J, Dent R, et al. Overall survival with pembrolizumab in early-stage triple-negative breast cancer. N Engl J Med. 2024;391(21):1981-1991.
- KEYTRUDA. Summary of product characteristics.
- NICE. Pembrolizumab for neoadjuvant and adjuvant treatment of triple-negative early or locally advanced breast cancer. Available at: https://www.nice.org.uk/guidance/ta851/chapter/1-Recommendations. Accessed: September 2025.
- The Scottish Medicines Consortium. Pembrolizumab (KEYTRUDA). Available at: https://scottishmedicines.org.uk/medicines-advice/pembrolizumab-keytruda-full-smc2247/. Accessed: September 2025.
Supporting documentation
Prescribing Information [External link]
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website.


