About ZERBAXA
About ZERBAXA® (ceftolozane/tazobactam)
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland) [External links]

For your critically ill adult patients who have ventilator-associated pneumonia due to confirmed or suspected Pseudomonas aeruginosa*2,3
*To be used in combination with an antibacterial agent active against Gram-positive pathogens when these are known or suspected to be contributing to the infectious process.1
Consideration should be given to official guidance on the appropriate use of antibacterial agents. Please refer to SmPC for a full list of indications, and before prescribing.
ZERBAXA is indicated for the treatment of adults with Hospital-Acquired Pneumonia (HAP), including Ventilator-Associated Pneumonia (VAP)1
ZERBAXA was studied in:
The recommended intravenous infusion (IV) dose of ZERBAXA with patients with creatinine clearance >50 mL/min is 3 g (2 g ceftolozane / 1 g tazobactam) administered every 8 hours by IV infusion over 1 hour, for treatment period of 8–14 days in patients 18 years or older with HAP (vHAP) including VAP.1**
**To be used in combination with an antibacterial agent active against Gram-positive pathogens when these are known or suspected to be contributing to the infectious process.
For full information on dose adjustments in adults with renal impairment (with creatinine clearance† ≤50 mL/min) and end stage renal disease, please refer to the Summary of Product Characteristics
No dose adjustment is necessary in patients with hepatic impairment
†Creatinine clearance estimated using Cockcroft-Gault formula.
Various pharmacokinetics (PK) factors are altered in critically ill patients and can have profound effects on the attainment of adequate antibiotic concentration4
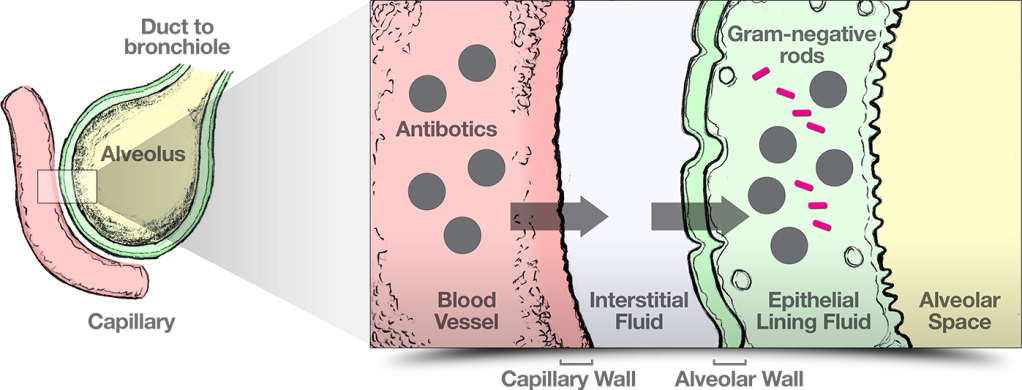
Adapted from Xiao AJ et al. 2016;5 Onufrak NJ et al. 2016;6 Rizk ML et al. 2018;7Välitalo PAJ et al. 2016.8
Attaining adequate concentration4
Patients may not attain MIC of pathogen and maximum bacterial killing, impacting outcomes.
Lung penetration, bronchopulmonary pharmacokinetic/pharmacodynamic profile and safety of 2 g/1 g of ceftolozane/tazobactam administered to ventilated, critically ill patients with pneumonia10
Ceftolozane individual and mean ELF and plasma concentration-time profile
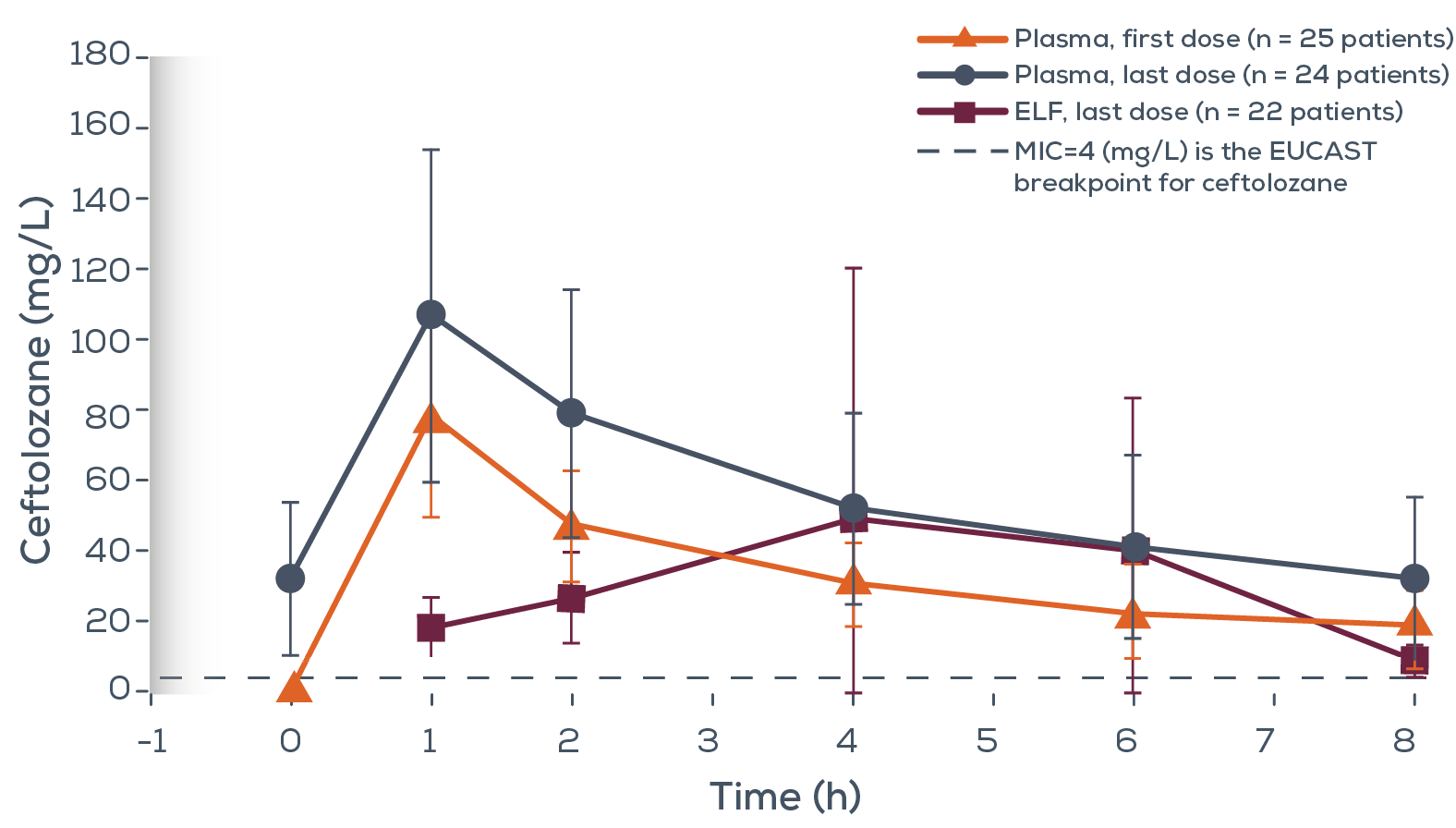
Adapted from Caro et al. 2020.10
Preliminary Phase 1 open label non-comparative PK study aimed to evaluate lung penetration of ceftolozane and tazobactam of 26 ventilated, critically ill patients* shows:10
* Patients received 4–6 doses of ceftolozane/tazobactam. Blood concentrations were obtained after the first and last dose; ELF concentrations were obtained 1, 2, 4, 6 and 8 hours after the last dose.
ZERBAXA was studied in critically ill patients in the ICU as part of assessment of the safety profile and efficacy of ceftolozane-tazobactam-nosocomial pneumonia (ASPECT-NP)2
Studied in a Phase 3, double–blind, multinational non–inferiority study
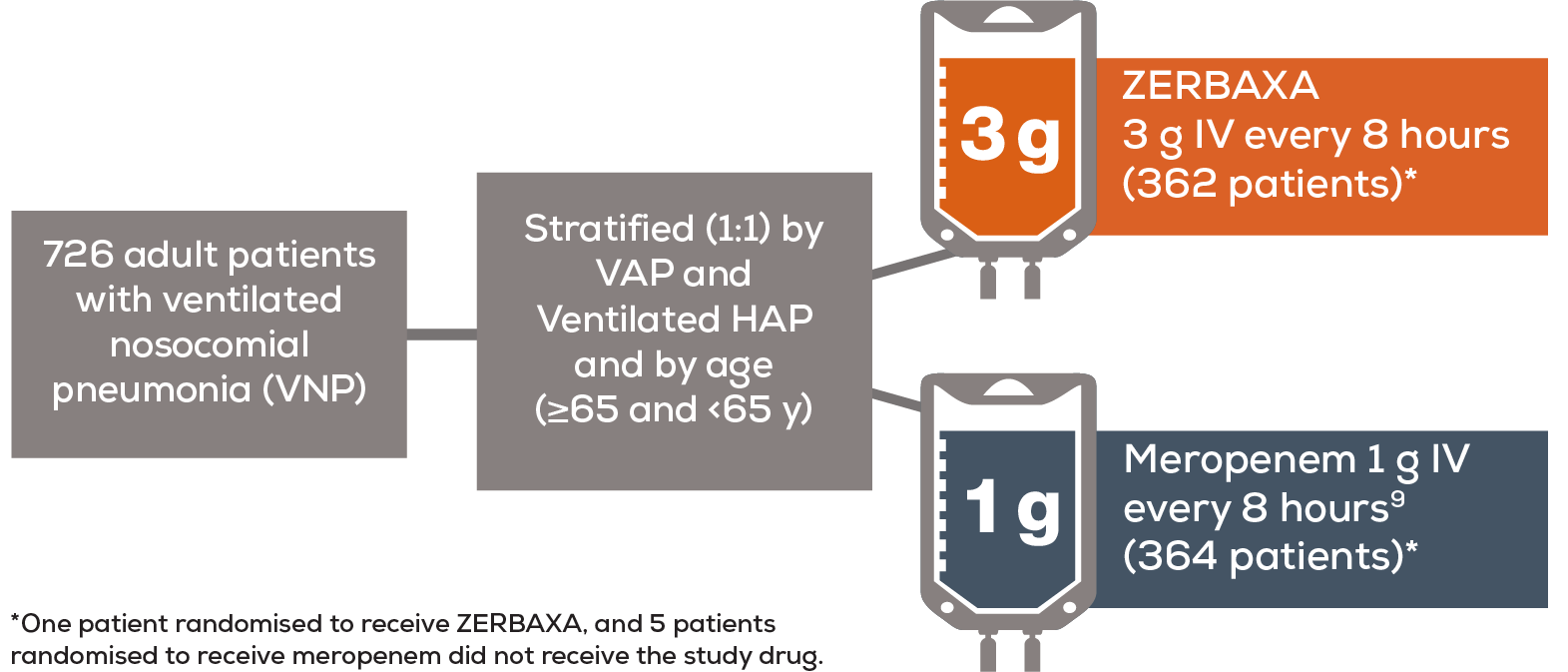
Study analysed intent-to-treat population which included all randomised patients.
European Medicines Agency (EMA) primary objective and endpoint11
Key secondary objective and endpoint11
Based on select baseline characteristics including:
Adapted from Kollef et al. 20192 and Data on File REF-4087811
Key inclusion and exclusion criteria
Adapted from Kollef et al. 2019.2
ZERBAXA achieved non-inferiority in clinical cure at TOC and 28-day all-cause mortality11
Results for primary and secondary endpoints and VAP and vHAP diagnosis within the non-inferiority margin for EMA11
Clinical cure rates at TOC in the ITT population Intent–to–treat population
Adapted from Data on File REF-4087011
*Pre-specified subgroup analyses gave exploratory endpoints for patients with VAP and vHAP, as well as individual baseline characteristics. No statistical conclusions can be drawn from exploratory endpoints.11
Safety and tolerability profile for HAP and VAP indications only
ZERBAXA was evaluated in a Phase 3 comparator-controlled clinical trial of hospital-acquired pneumonia, including ventilator-associated pneumonia.1
The most common adverse reactions (≥ 5% in a Phase 3 trial of hospital-acquired pneumonia, including ventilator-associated pneumonia) occurring in patients receiving ZERBAXA were diarrhoea, alanine aminotransferase increased, and aspartate aminotransferase increased and were generally mild or moderate in severity1
Adverse reactions identified during clinical trials with ceftolozane/tazobactam1
| System organ class | Common (≥1/100 to <1/10 ) | Uncommon (≥1/1,000 to <1/100 ) |
|---|---|---|
| Infections and infestations | Clostridioides difficile colitis* | Clostridioides difficile infection* |
| Gastrointestinal disorders | Diarrhoea**, vomiting** | – |
| Investigations | Alanine aminotransferase increased,** aspartate aminotransferase increased,** transaminases increased*, liver function test abnormal,* blood alkaline phosphatase increased,* gamma‑glutamyltransferase increased* | Coombs test positive,** Clostridioides test positive* |
Limitations of the clinical data: Patients who were immunocompromised, and patients with severe neutropenia, and patients with end‑stage renal disease on haemodialysis were excluded from clinical trials.
Please see ZERBAXA Summary of Product Characteristics for full details of adverse reactions identified during clinical trials with ceftolozane/tazobactam including those of other indications.
*Specific for the hospital-acquired pneumonia, including ventilator-associated pneumonia indication treated with ZERBAXA (2 g / 1 g intravenously every 8 hours) for up to 14 days.
**Applies across all indications: complicated intra-abdominal infections, acute pyelonephritis, complicated urinary tract infections, and hospital-acquired pneumonia, including ventilator-associated pneumonia.
Abbreviations
APACHE II = Acute Physiology and Chronic Health Evaluation II; ASPECT-NP = Assessment of the Safety Profile and Efficacy of Ceftolozane-Tazobactam-Nosocomial Pneumonia; ELF = Epithelial Lining Fluid; EMA = European Medicines Agency; EOT = End of Therapy; FDA = Food and Drug Administration; HAP = Hospital-Acquired Pneumonia; ITT = Intent-To-Treat; MIC = Minimum Inhibitory Concentration; NP = Nosocomial Pneumonia; PD = Pharmacodynamic; PK = Pharmacokinetics; TOC = Test-Of-Cure; VAP = Ventilator-Associated Pneumonia; vHAP = Ventilated Hospital-Acquired Pneumonia; VNP = Ventilated Nosocomial Pneumonia.
References
ZERBAXA® is indicated for the treatment of the following infections in adults: complicated intra-abdominal infections; acute pyelonephritis; complicated urinary tract infections, hospital-acquired pneumonia including ventilator-associated pneumonia. Consider official guidance on the appropriate use of antibacterial agents.
CONTRAINDICATIONS
Hypersensitivity to active substances or to any of the excipients; hypersensitivity to any cephalosporin antibacterial agent; severe hypersensitivity (e.g., anaphylactic reaction, severe skin reaction) to any other type of beta lactam antibacterial agent (e.g., penicillins or carbapenems).
PRECAUTIONS
Hypersensitivity reactions: serious and occasionally fatal hypersensitivity (anaphylactic) reactions are possible. In case of severe allergic reaction during treatment, discontinue and take appropriate measures. Patients with history of hypersensitivity to cephalosporins, penicillins or other beta-lactam antibacterial agents may also be hypersensitive to ceftolozane/tazobactam. Use with caution in patients with a history of any other type of hypersensitivity reaction to penicillins or other beta lactam antibacterial agents. Effect on renal function: a decline in renal function has been seen in patients receiving ceftolozane/tazobactam. Impaired renal function: adjust ceftolozane/tazobactam dose based on renal function. In clinical trials of complicated intra-abdominal infections and urinary tract infections, including pyelonephritis, the efficacy of ceftolozane/tazobactam was lower in patients with moderate renal impairment compared with those with normal or mildly impaired renal function at baseline. Monitor patients with renal impairment at baseline frequently for any changes in renal function during treatment and adjust dose as necessary. Clostridioides difficile associated diarrhoea: antibacterial-associated colitis and pseudomembranous colitis have been reported, ranging in severity from mild to life threatening. Consideration of this diagnosis is important in patients who present with diarrhoea during or after the administration of ceftolozane/tazobactam. In such cases, consider discontinuation of and use of supportive measures together with administration of specific treatment for Clostridioides difficile. Non-susceptible microorganisms: overgrowth may be promoted by use of ceftolozane/tazobactam. In case of super infection during or following treatment, take appropriate measures. Ceftolozane/tazobactam is not active against bacteria that produce beta lactamase enzymes which are not inhibited by tazobactam. Direct antiglobulin test (Coombs test) seroconversion and potential risk of haemolytic anaemia: development of a positive direct antiglobulin test (DAGT) may occur during treatment. In clinical studies, there was no evidence of haemolysis in patients who developed a positive DAGT on treatment. Sodium content: ceftolozane/tazobactam contains 230 mg of sodium per vial. The reconstituted vial with 10 mL of 0.9% sodium chloride (normal saline) for injection contains 265 mg of sodium.
INTERACTION WITH OTHER MEDICINAL PRODUCTS AND OTHER FORMS OF INTERACTION
No significant interactions anticipated between ceftolozane/tazobactam and substrates, inhibitors, and inducers of cytochrome P450 enzymes (CYPs) based on in vitro and in vivo studies. Tazobactam is a substrate for OAT1 and OAT3. In vitro, tazobactam inhibited human OAT1 and OAT3 transporters with IC50 values of 118 and 147 mcg/mL, respectively. Co-administration of ceftolozane/tazobactam with OAT1 and OAT3 substrate furosemide in a clinical study did not significantly increase furosemide plasma exposures (geometric mean ratios of 0.83 and 0.87 for Cmax and AUC, respectively). Active substances that inhibit OAT1 or OAT3 (e.g., probenecid) may increase tazobactam plasma concentrations.
FERTILITY, PREGNANCY AND LACTATION
Pregnancy: no data in humans. Tazobactam crosses the placenta. It is not known if ceftolozane crosses the placenta. Use only if expected benefit outweighs possible risks to the pregnant woman and foetus. Breastfeeding: unknown whether ceftolozane and tazobactam are excreted in human milk and risk to newborns/infants cannot be excluded. Either discontinue breastfeeding or discontinue/abstain from ZERBAXA® therapy taking into account benefit of breastfeeding for the child and benefit of therapy for the woman. Fertility: not studied in humans.
UNDESIRABLE EFFECTS
Refer to SmPC for complete information on side effects. The following adverse reactions have been identified during clinical trials with ZERBAXA®. Frequency categories are derived according to the following conventions: common (≥ 1/100 to < 1/10), uncommon (≥ 1/1,000 to < 1/100). Complicated intra-abdominal infections, acute pyelonephritis, and complicated urinary tract infections indications:
- Common: Clostridioides difficile colitis, transaminases increased, liver function test abnormal, blood alkaline phosphatase increased, gamma-glutamyl transferase increased
- Uncommon: Clostridioides difficile infection, Clostridioides test positive.
- Common: diarrhoea, vomiting, alanine aminotransferase increased, aspartate aminotransferase increased
- Uncommon: Coombs test positive
Hospital-acquired pneumonia and ventilator-associated pneumonia indication:
Applies across all indications:
Supporting documentation
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland)
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website
