Pulmonary Arterial Hypertension
PAH Management Considerations
Assessments that may be used in clinical monitoring and determining risk status
WHO=World Health Organization.
References: 1. Humbert M et al. Eur Heart J. 2022;43(38):3618-3731. 2. McLaughlin VV et al. In: Zipes DP et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 11th ed. Elsevier; 2019:1699-1719. 3. Maron BA. In: Libby P et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 12th ed. Elsevier; 2022:1656-1677.
About PAH
The World Health Organization (WHO), through the proceedings of the World Symposium on Pulmonary Hypertension (WSPH), provided an updated clinical classification of PH in 20191,2
Pulmonary arterial hypertension (PAH) is classified as group 1 pulmonary hypertension (PH)1,2
WHO Group Classification1,2
PAH is characterised by pre-capillary pulmonary hypertension in the absence of other causes, such as chronic thromboembolic pulmonary hypertension and pulmonary hypertension associated with lung diseases.1
Severity of PH is measured using the WHO functional classification1
There are 4 WHO functional classes (FCs), which reflect increasing severity of disease and symptom burden1
WHO categorises PH into 4 FCs
PH is a disorder defined by a mean pulmonary arterial pressure >20 mm Hg at rest.1
PAH is a rare, chronic and progressive disease1,3
In a systematic review of 15 studies, including 13 registries and 8 databases, published between 2003 and 2020:4
- PAH affected more female (~55%-81%) vs male (~20%-45%) patients, and the mean age of patients with PAH ranged from 43 to 67 years, depending on the country4,5
- Based on registry data from 2022, the prevalence of PAH in the UK is estimated to be between 55-60 per million adults1,4a
aThe selected publications were from 13 European, 3 Asian, and 2 North American countries, as well as 1 South American country.4
3-year mortality rates for 6580 adult patients diagnosed with PAH by WHO-FC as shown in a systematic review of 10 observational registry studies published between 2001 and 2018:3
The 3-year mortality rate for patients with WHO-FC I/II was lower than for those patients with WHO-FC III/IV.3
PAH has numerous aetiologies, including genetic, pathogen-related causes and others6
Registry data revealed that the most common aetiologies of PAH in the UK were IPAH (33%), Congential Heart disease APAH (33%), connective tissue disease APAH (24%), Portal hypertension associated APAH (4%)7
PAH can be:6
- Idiopathic
- Heritable
- Induced by drugs or toxins
- Associated with other conditions, such as CTD, HIV infection, portal hypertension, CHD, and schistosomiasis
Learn more about PAH disease progression and its impact on the heart
CHD=congenital heart disease; CI=confidence interval; CTD=connective tissue disease; HF=heart failure; HIV=human immunodeficiency virus; QUERI=Quality Enhancement Research Initiative; REVEAL=Registry to Evaluate Early and Long-Term PAH Disease Management; UK=United Kingdom.
References: 1. Humbert M et al. Eur Heart J. 2022;43(38):3618-3731. 2. Simonneau G et al. Eur Respir J. 2019;53(1):1-13. 3. Kim NH et al. Pulm Circ. 2020;10(4):1-10. 4. Leber L et al. Pulm Circ. 2021;11(1):1-12. 5. Leber L et al. Pulm Circ. 2021;11(1)(suppl):S1-S26. 6. Hoeper MM et al. Lancet Respir Med. 2016;4(4):306-322. 7. 2021-2022 Uk National registry: https://files.digital.nhs.uk/36/B8B717/NAPH%2013AR%20-%20Main%20Report%20v1.0.pdf.
PAH Pathophysiology
Pulmonary arterial hypertension (PAH) has a complex pathophysiology1-4
There are many factors that may contribute to the complex pathophysiology in PAH, including the following:1-6
- An imbalance in vasodilation and vasoconstriction
- Cell dysfunction
- Thrombosis
- Inflammation
Pulmonary vascular changes in PAH are characterised by abnormalities, such as intimal hyperproliferation, medial hyperplasia and hypertrophy, apoptosis resistance, thrombosis, adventitia thickening, and inflammatory cell influx1-3
Pulmonary vasculopathy1,3
The net effects of these abnormalities are:1
- Vascular stiffening
- Endothelial dysfunction
- Inflammation
- Thrombosis
- Vascular obstruction
- Vasoconstriction
- Excessive fibrosis
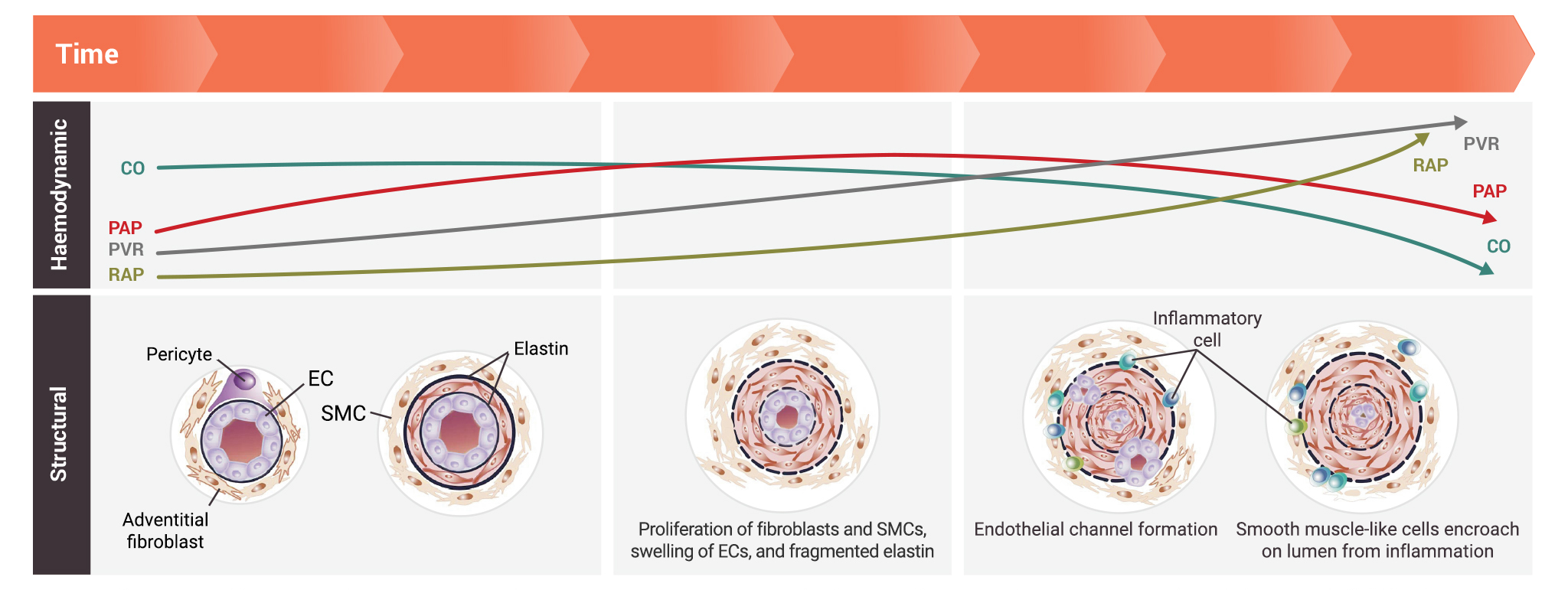
Pathogenic changes in the architecture of pulmonary arteries may lead to increased pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR) over time1
The pulmonary vascular bed is densely packed with blood vessels that maximise surface area for gas exchange. Changes in the structure of pulmonary arteries may lead to early decline in pulmonary arterial compliance prior to rise in PVR and pulmonary artery blood pressure.1
Turn screen horizontally to expand for larger view on mobile device
Adapted with permission of the American Thoracic Society. Copyright © 2023 American Thoracic Society. All rights reserved. Maron BA, Abman SH. 2017. Translational advances in the field of pulmonary hypertension. Focusing on developmental origins and disease inception for the prevention of pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 195(3):292-301. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society. Readers are encouraged to read the entire article for the correct context at https://www.atsjournals.org/doi/10.1164/rccm.201604-0882PP?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.7
- Pathologic changes in the earlier stages of disease progression include:1
- Proliferation of fibroblasts and smooth muscle cells
- Swelling of endothelial cells
- Presence of fragmented elastin
- Pathologic changes in the later stages of disease progression include:1
- Presence of inflammatory cells
- Endothelial channel formation
- Encroachment of smooth muscle-like cells on the lumen from inflammation
As PAH progresses, it has an impact on the right side of the heart2,8-10
In PAH, pulmonary vascular remodelling and sustained vasoconstriction narrow the pulmonary arteries2,8,11
To overcome the increasing PAP and PVR, along with RV afterload, the RV undergoes adaptive remodelling8,10
Adapted with permission from Rosenkranz S et al. Circulation. 2020;141(8):678-693.13
RV remodelling and severe hypertrophy ultimately lead to right heart dysfunction and failure8,10
Learn more about patient risk assessment and prognostic tools
CO=cardiac output; EC=endothelial cell; LV=left ventricle; RAP=right atrial pressure; RV=right ventricle; SMC=smooth muscle cell.
References: 1. Maron BA. In: Libby P et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 12th ed. Elsevier; 2022:1656-1677. 2. McLaughlin VV et al. In: Zipes DP et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 11th ed. Elsevier; 2019:1699-1719. 3. Humbert M et al. Eur Heart J. 2022;43(38):3618-3731. 4. Kurakula K et al. Biomedicines. 2021;9:57. 5. Humbert M et al. Eur Respir J. 2019;24:1801887. 6. Morrell NW et al. Eur Respir J. 2019;24:1801899. 7. Maron BA et al. Am J Respir Crit Care Med. 2017;195(3):292-301. 8. Thompson AAR et al. Trends Mol Med. 2017;23(1):31-45. 9. Vonk Noordegraaf A et al. Eur Respir Rev. 2011;20(122):243-253. 10. Wang Z et al. Pulm Circ. 2011;1(2):212-223. 11. Tuder RM et al. J Am Coll Cardiol. 2013;62(25)(suppl D):D4-D12. 12. Keen J et al. Front Physiol. 2021;11:1-11. 13. Rosenkranz S et al. Circulation. 2020;141(8):678-693.
PAH Management Considerations
Multiple assessments can be used at diagnosis of PAH to estimate prognosis
Variables used at diagnosis in the 3-strata risk-assessment tool1
Turn screen horizontally to expand for larger view on mobile device
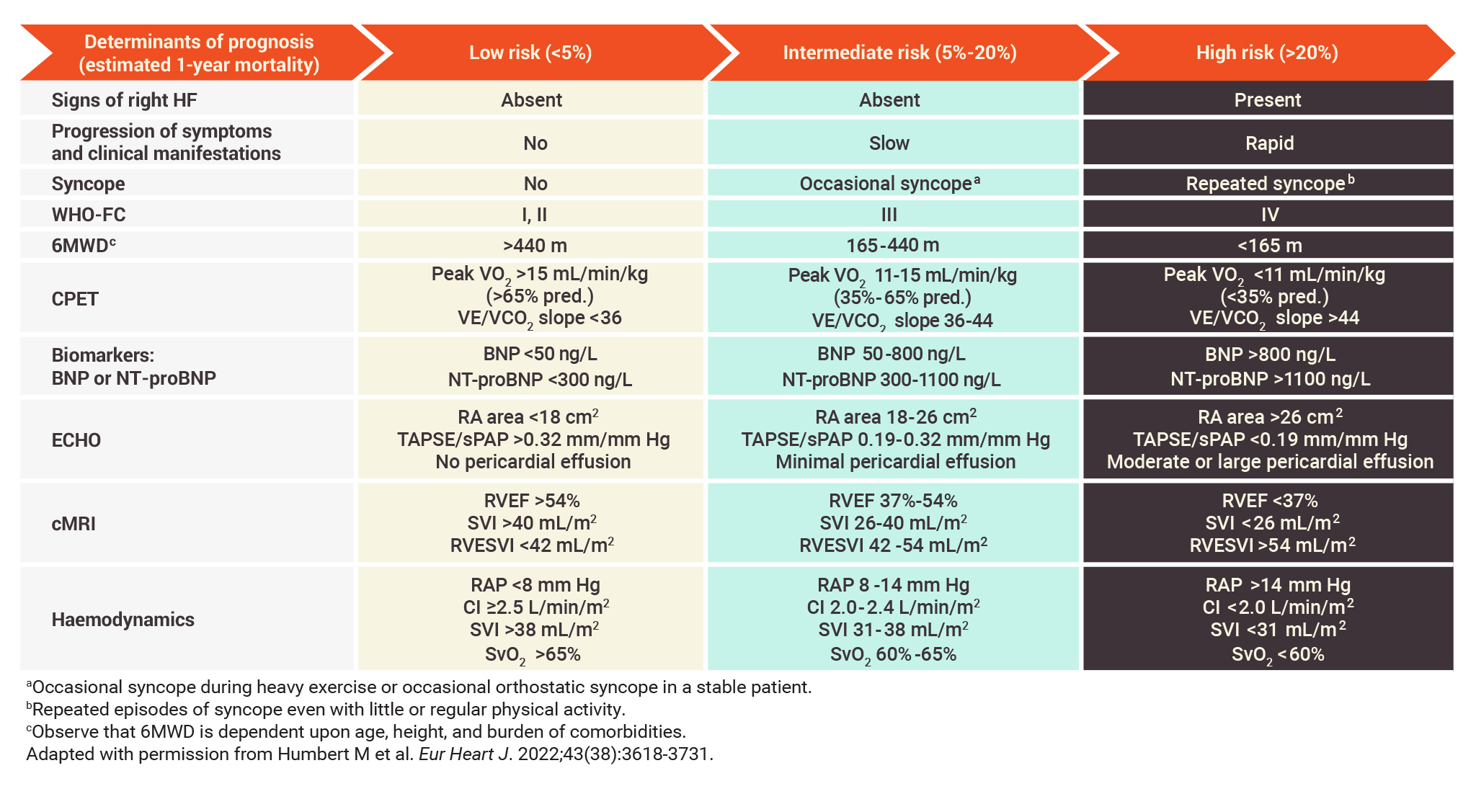
- The parameters used in the 3-strata risk-assessment tool include signs of right HF, symptom progression and clinical manifestations, syncope, WHO-FC, 6MWD, CPET, biomarker levels, echocardiography, cMRI, and haemodynamic parameters1
- These assessments are among those that registry analyses and studies have identified to have value in establishing severity, stability, and prognosis in PAH2-6
Changes in risk status at follow-up can be detected using the more sensitive 4-strata model1,7
Variables used at follow-up in the simplified 4-strata risk-assessment tool1
Turn screen horizontally to expand for larger view on mobile device
- This 4-strata risk-assessment tool based on refined cutoffs for select assessments may be more sensitive to prognostically relevant changes in risk than the original 3-strata model, including increased sensitivity to changes associated with variations in the patients’ long-term mortality risk1,7
Learn more about PAH, WHO classification, and PAH aetiology
6MWD=6-minute walking distance; 6MWT=6-minute walking test; ABG=arterial blood gas analysis; ALAT=alanine aminotransferase; ASAT=aspartate aminotransferase; BNP=brain natriuretic peptide; CHEST=American College of Chest Physicians; CI=cardiac index; cMRI=cardiac magnetic resonance imaging; COMPERA=Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension; CPET=cardiopulmonary exercise testing; DLCO=diffusing capacity of the lungs for carbon monoxide; ECG=electrocardiogram; ECHO=echocardiogram; FPHR=French Pulmonary Hypertension Registry; HF=heart failure; HR-QoL=health-related quality of life; INR=international normalised ratio; NT-proBNP=N-terminal pro-brain natriuretic peptide; PAP=pulmonary arterial pressure; PH=pulmonary hypertension; PHIRST=Pulmonary Arterial Hypertension and Response to Tadalafil; pred.=predicted; prog. symptoms=progression of symptoms; PVR=pulmonary vascular resistance; RA=right atrium; RAP=right atrial pressure; REVEAL=Registry to Evaluate Early And Long-Term PAH Disease Management; RHC=right heart catheterisation; RHF=right heart failure; RVEF=right ventricular ejection fraction; RVESVI=right ventricular end-systolic volume index; SBP=systolic blood pressure; SPAHR=Swedish Pulmonary Arterial Hypertension Registry; sPAP=systolic pulmonary arterial pressure; SVI=stroke volume index; SvO2=mixed venous oxygen saturation; TAPSE=tricuspid annular plane systolic excursion; TRIUMPH=Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension; TSH=thyroid-stimulating hormone; VE/VCO2=ventilatory equivalent for carbon dioxide; VO2=oxygen uptake; WHO-FC=World Health Organization functional class; WSPH=World Symposium on Pulmonary Hypertension.
References: 1. Humbert M et al. Eur Heart J. 2022;43(38):3618-3731. 2. Dardi F et al. Open Heart. 2021;8(2):1-10. 3. Nickel N et al. Eur Respir J. 2012;39(3):589-596. 4. Heresi GA et al. Lung. 2020;198(6):933-938. 5. Kylhammar D et al. Eur Heart J. 2018;39(47):4175-4181. 6. Hoeper MM et al. Eur Respir J. 2017;50(2):1-10. 7. Hoeper MM et al. Eur Respir J. 2022;60(1):1-12.

