VAXNEUVANCE in adults ≥18 years
VAXNEUVANCE®▼ (pneumococcal polysaccharide conjugate vaccine (15-valent, adsorbed)) can help protect your adult patients against 15 pneumococcal serotypes1-3
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland) [External links]
VAXNEUVANCE is indicated for active immunisation for the prevention of invasive disease, pneumonia and acute otitis media caused by Streptococcus pneumoniae in infants, children and adolescents from 6 weeks to less than 18 years of age.1,2
VAXNEUVANCE is indicated for active immunisation for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older.1,2
Please refer to the VAXNEUVANCE SmPC for further information before making any prescribing decisions. The use of VAXNEUVANCE should be in accordance with official recommendations.1,2
VAXNEUVANCE contains two additional Streptococcus pneumoniae serotypes versus PCV13
(13-valent pneumococcal conjugate vaccine)1,2,4
PCV13 contains 13 Streptococcus pneumoniae serotypes4


Study design
PNEU-AGE was a Phase 3, multicentre, randomised, double-blind, active comparator-controlled study conducted from June 2019 through to March 2020 at 30 sites.
| N=1,202 adult patients | Stratification | Randomised 1:1 | Primary endpoints |
|---|---|---|---|
| Inclusion criteria: ● Male or female participants in generally good health and/or with stable underlying medical conditions ≥50 years of age (or for sites in Japan, ≥65 years of age) | Participant age at enrollment 50–64 years 65–74 years ≥75 years | V114 (VAXNEUVANCE) single dose (n=602) | Proportion of participants with: ● Solicited injection-site AEs from pre-vaccination (Day 1) through Day 5 post-vaccination ● Solicited systemic AEs from Day 1 through Day 14 post-vaccination ● The broad AE categories of any AE, any SAE or any vaccine-related SAE Non-inferiority of immune responses for shared PCV13 STs and superiority of immune response for STs unique to V114 |
| Key exclusion criteria: ● History of IPD or other culture positive pneumococcal disease within the previous 3 years ● Known hypersensitivity to a vaccine component ● Known or suspected impairment of immunological function ● Prior receipt of any pneumococcal vaccine | Participant age at enrollment 50–64 years 65–74 years ≥75 years | PCV13 single dose (n=600) | Secondary endpoints ● Superiority of immune response for ST 3 via the OPA GMTs at 30 days post-vaccination ● Proportions of participants with a ≥4-fold rise from Day 1 to 30 days post-vaccination (Day 30) ● ST-specific specific IgG GMCs at 30 days following vaccination with V114 compared to PCV13 ● Observed ST-specific OPA GMTs and IgG GMCs at 30 days post-vaccination ● GMFR and proportions of participants with a ≥4-fold rise from Day 1 to 30 days post-vaccination (Day 30) for both OPA and IgG responses |
Table adapted from Platt HL, et al. Vaccine 2022.
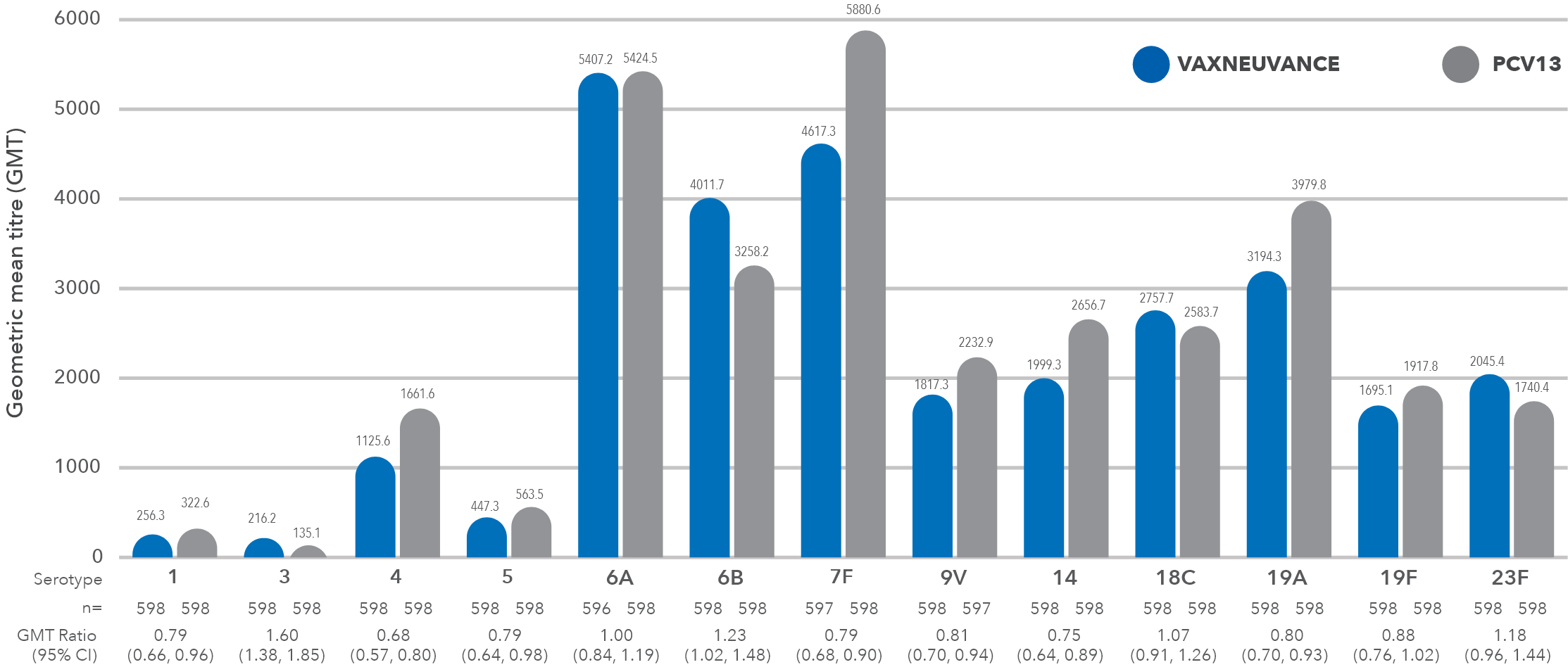
In the pivotal PNEU-AGE study in vaccine-naïve adults ≥50 years, VAXNEUVANCE demonstrated:1-3
- Non-inferior immune responses for 13 serotypes shared with PCV133
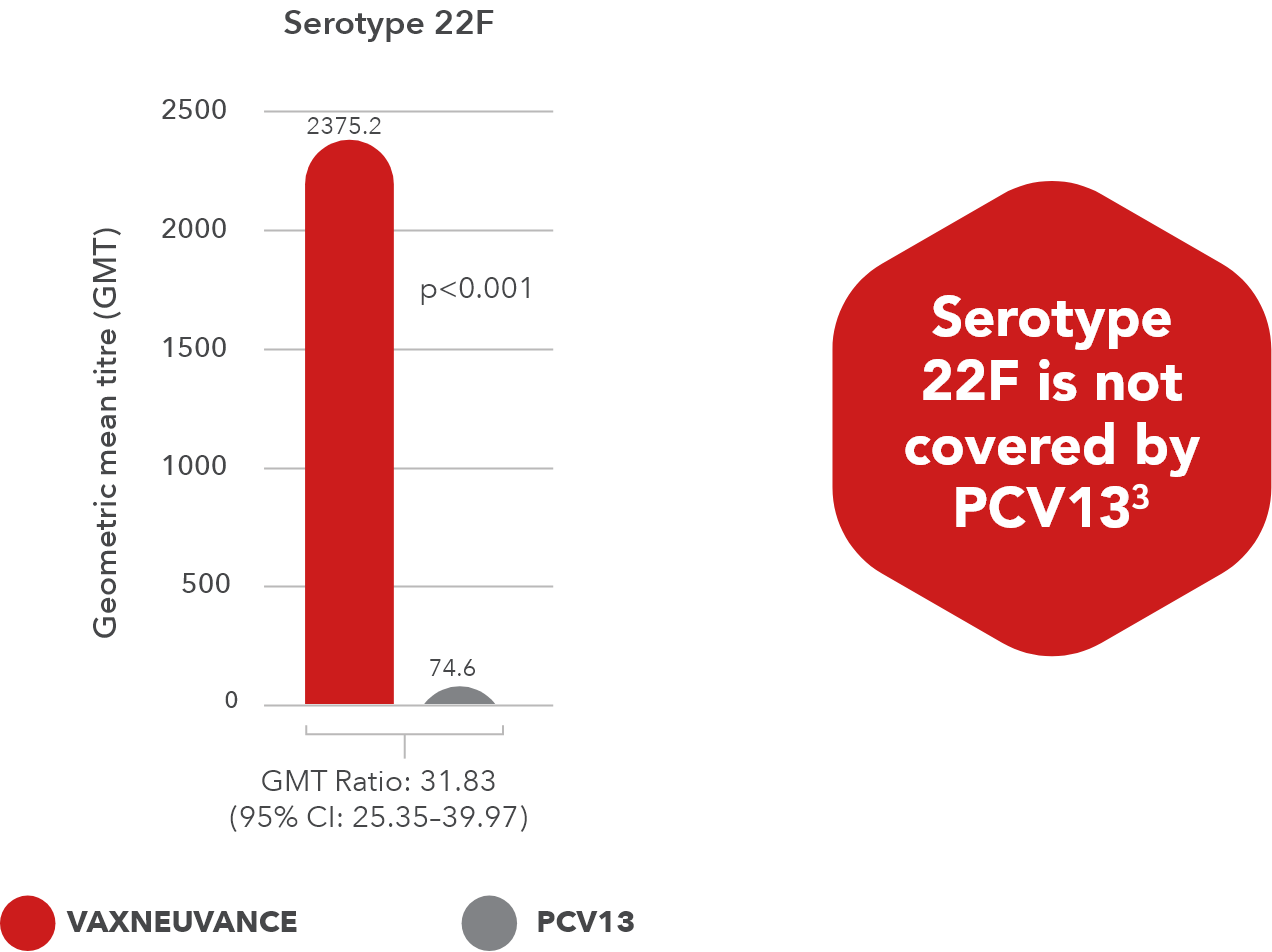
- Superior immune responses for two additional serotypes vs. PCV13: 22F and 33F3
- Serotype 22F: p<0.001, GMT ratio 31.83, 95% CI: 25.35-39.97
- Serotype 33F: p<0.001, GMT ratio 7.11, 95% CI: 6.07-8.32
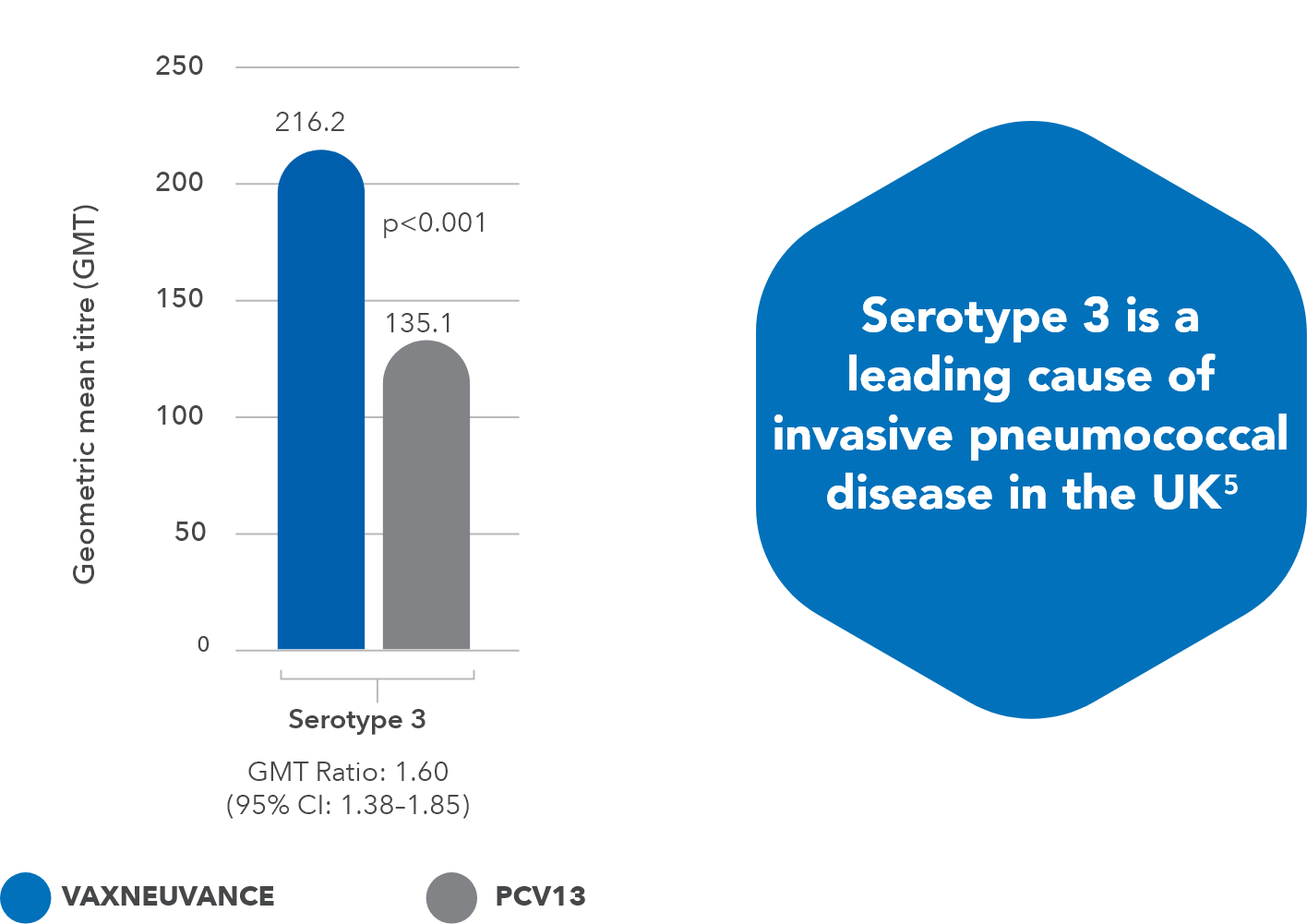
- Superior immune response vs. PCV13 for serotype 33
- Serotype 3: p<0.001, GMT ratio 1.60, 95% CI: 1.38-1.85
Serotype-specific comparative OPA GMTs 30 days post-vaccination
Graph adapted from Platt HL, et al. Vaccine 2022
Serotype-specific comparative OPA GMTs 30 days post-vaccination
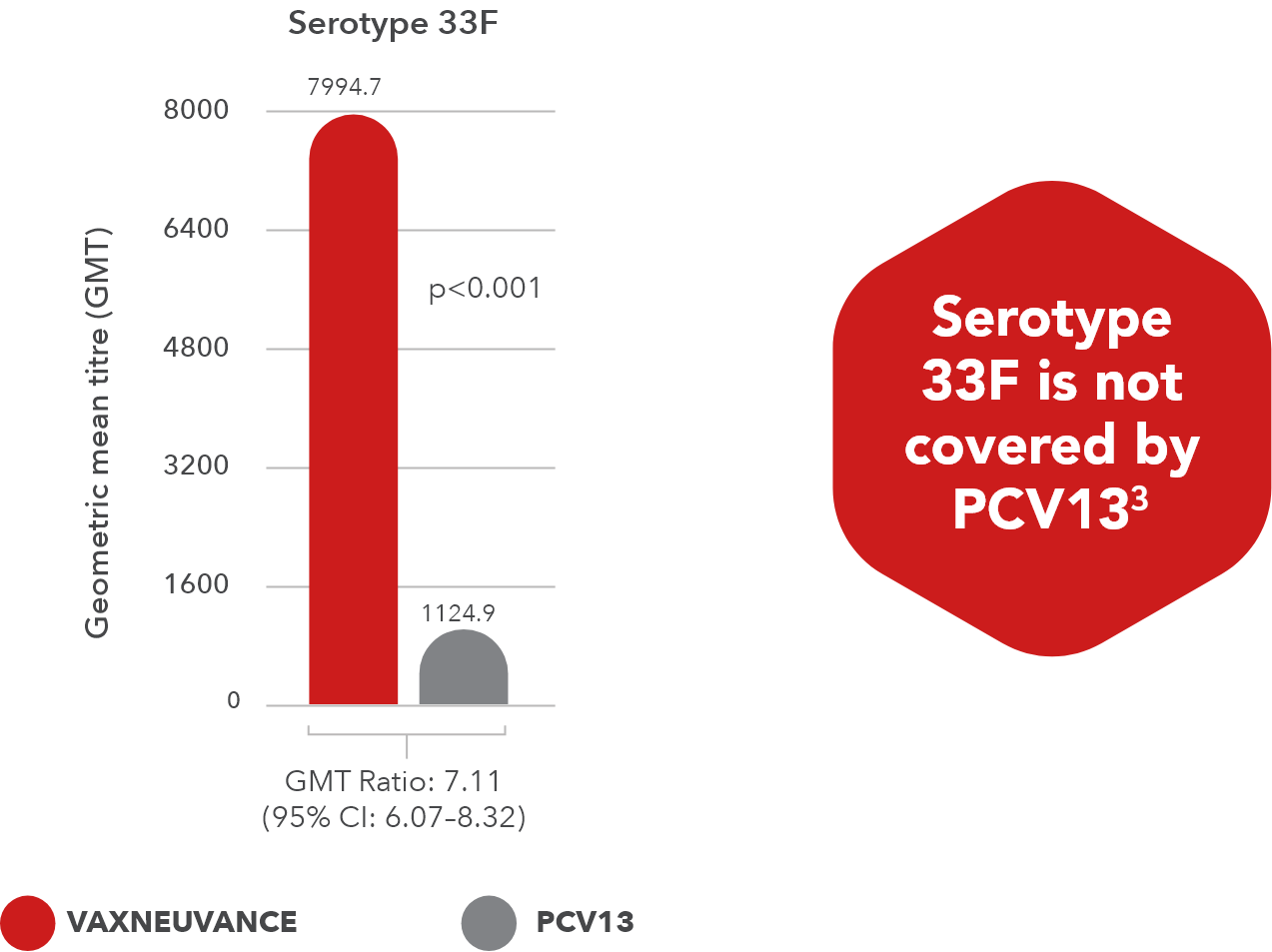
Graph adapted from Platt HL, et al. Vaccine 2022
AE = Adverse Event; GMC = Geometric Mean Concentration; GMF = Geometric Mean Fold rise; GMT = Geometric Mean Titre; IgG = Immunoglobulin G; IPD = Invasive Pneumococcal Disease; OPA = Opsonophagocytic Activity; PCV13 = 13-Valent Pneumococcal Conjugate Vaccine; SAE = Serious Adverse Event; V114 = VAXNEUVANCE 15-Valent Pneumococcal Conjugate Vaccine.
References
- VAXNEUVANCE Summary of Product Characteristics for GB.
- VAXNEUVANCE Summary of Product Characteristics for NI.
- Platt HL, et al. A phase 3 trial of safety, tolerability, and immunogenicity of V114, 15-valent pneumococcal conjugate vaccine, compared with 13-valent pneumococcal conjugate vaccine in adults ≥50 years of age and older (PNEU-AGE). Vaccine. 2022;40:162–172.
- Mohapi L, et al. Safety and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, in adults living with HIV: a randomised phase 3 study. AIDS. 2022 Mar 1;36(3):373–382.
- Hyams C, et al. Serotype Distribution and Disease Severity in Adults Hospitalized with Streptococcus pneumoniae Infection, Bristol and Bath, UK, 2006‒2022. Emerging Infectious Diseases. 2023;29(10):1953-1964.
Supporting documentation
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland)
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website.