Who is the adjuvant RCC patient?
KEYTRUDA® (pembrolizumab)
Who is the adjuvant RCC patient?
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland) [External links]
If your patient meets ANY ONE of these criteria following nephrectomy, consider KEYTRUDA instead of active surveillance alone. 2
Intermediate-high risk
pT2 with Grade 4 or sarcomatoid, N0, M0
pT3, any grade, N0, M0
High risk
pT4, any grade, N0, M0
Any pT, any grade with node positive, M0
M1 NED
M1
No evidence of disease (NED) after resection of oligometastatic sites ≤1 year from nephrectomy

KEYTRUDA reduced the risk of disease recurrence or death vs. placebo after nephrectomy2,3
Kaplan-Meier estimate of disease-free survival in KEYNOTE-564 (primary endpoint met, ITT population, 24.1 month median follow-up)2
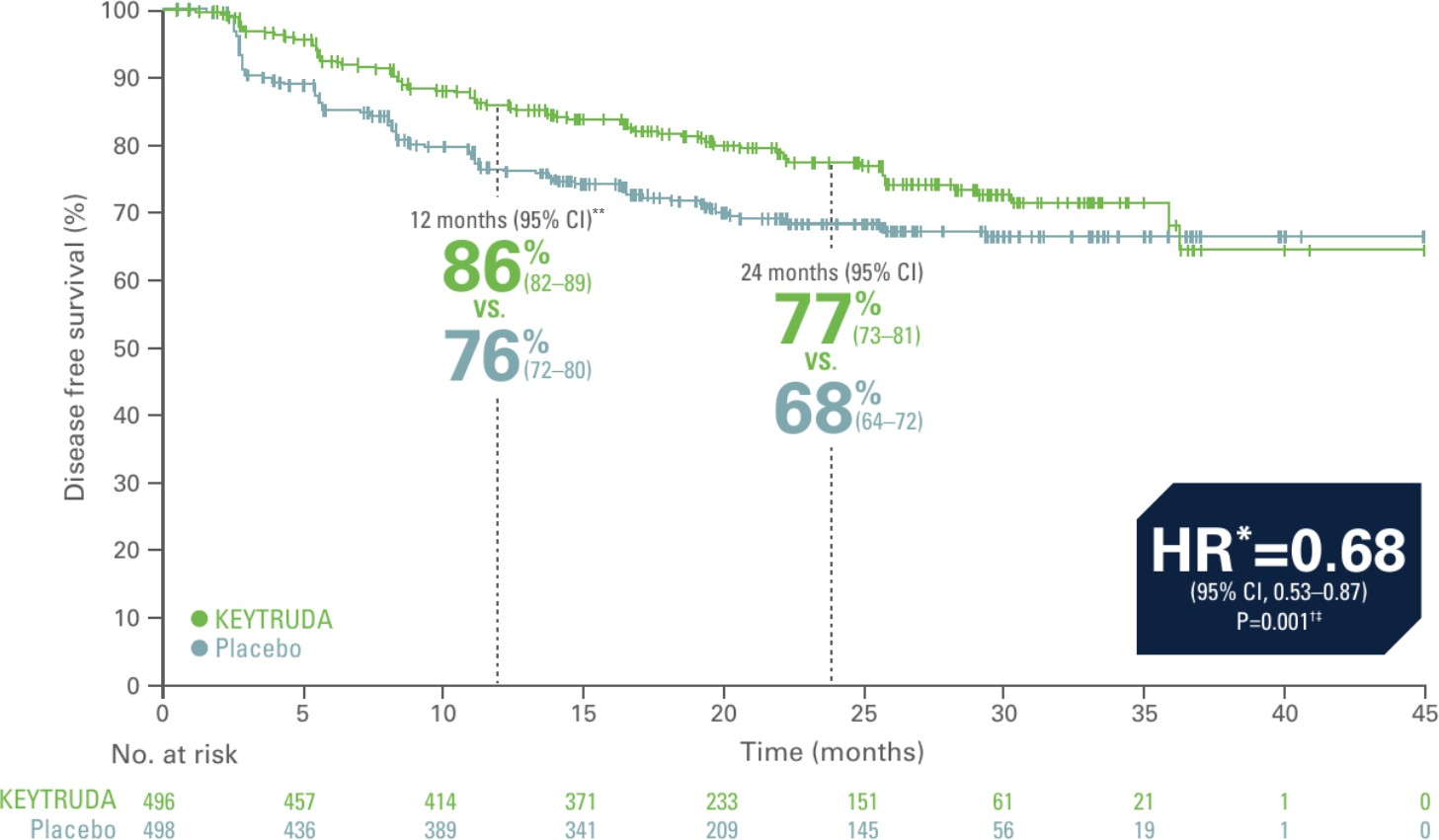
Adapted from Choueri TK et al. N Engl J Med. 2021;385(8):683–694.
- 32% reduction in the risk of disease recurrence or death with KEYTRUDA vs. placebo2
- Events observed: 109/496 (22%) with KEYTRUDA vs. 151/498 (30%) with placebo. Of these 260 events, 51 deaths had occurred and 209 patients had disease recurrence2
- Median DFS: not reached for either trial arm2
- 12-month DFS rate: 86% (95% CI, 82–89) with KEYTRUDA vs. 76% (95% CI, 72–80) with placebo2
- 24-month DFS rate: 77% (95% CI, 73–81) with KEYTRUDA vs. 68% (95% CI, 63–72) with placebo2
Primary analysis data cutoff date: December 14 2020.2
*Based on the stratified Cox proportional hazard model.2
**Time point was not part of the prespecified analysis and therefore a statistically significant conclusion cannot be drawn.
†Based on stratified log-rank test.2
‡P-value boundary for significance was 0.0114 (one-sided).2
Kaplan-Meier estimate of disease-free survival in KEYNOTE-564 (ITT population, 30.1 month median follow-up)3*
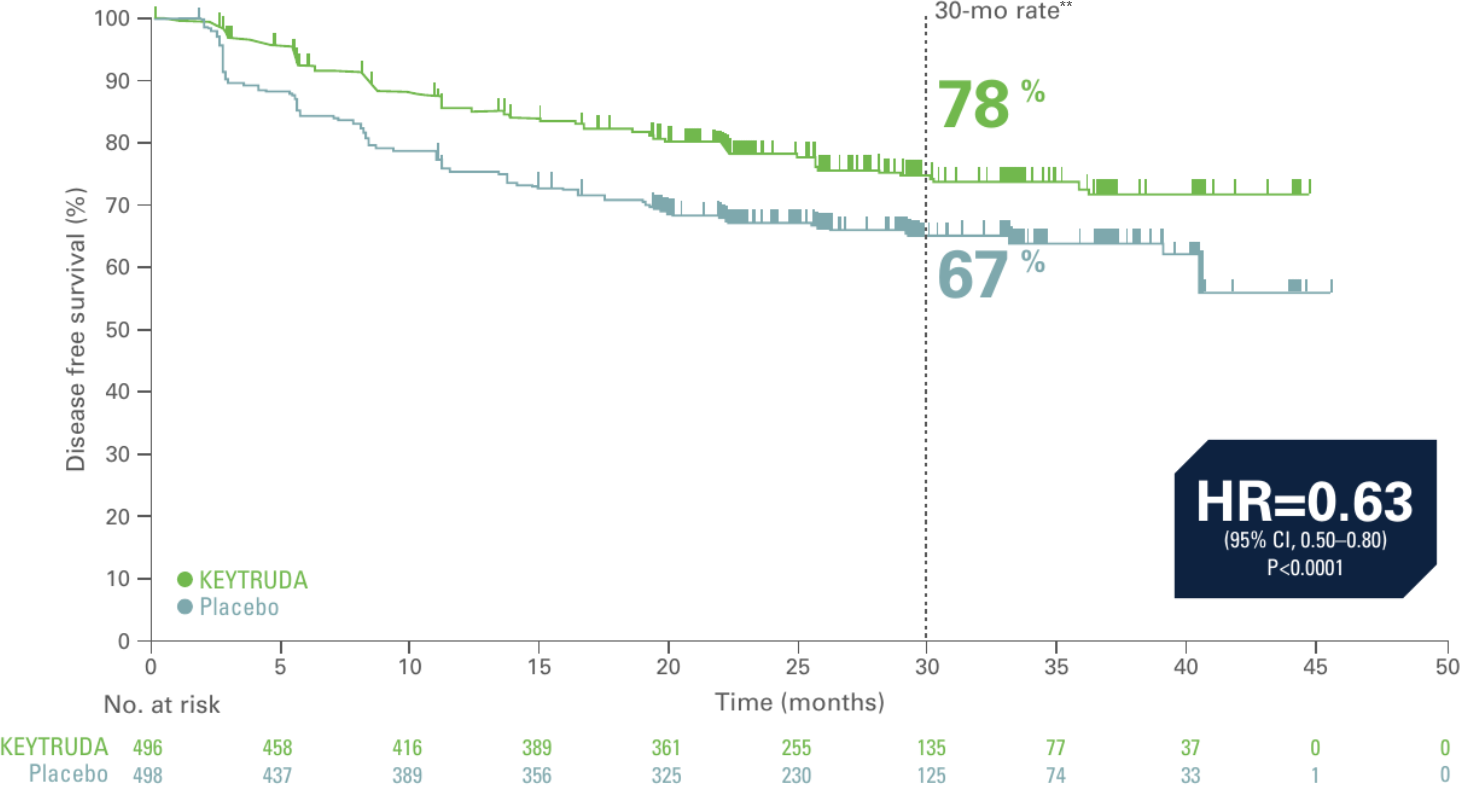
Adapted from Choueri TK et al. Presented at ASCO 2022. J Clin Oncol. 2022;40:6 suppl:290-290.
- 37% reduction in the risk of disease recurrence or death with KEYTRUDA vs. placebo3
- Events observed: 114/496 (23%) with KEYTRUDA vs. 168/498 (34%) with placebo3
- Median DFS: Not reached for either trial arm3
Updated analysis data cutoff: June 14, 2021. This was an unplanned update to demonstrate the continued efficacy of KEYTRUDA.3
*At the time of analysis, OS results were not yet mature, as only 33% of deaths needed for the final analysis had accrued at data cutoff.3
**Time point was not part of the prespecified analysis and therefore a statistically significant conclusion cannot be drawn.
Interim overall survival with KEYTRUDA vs. placebo after nephrectomy2,3
Kaplan-Meier estimate of overall survival in interim analysis of KEYNOTE-564 (secondary endpoint, ITT population, 24.1 month median follow-up)2
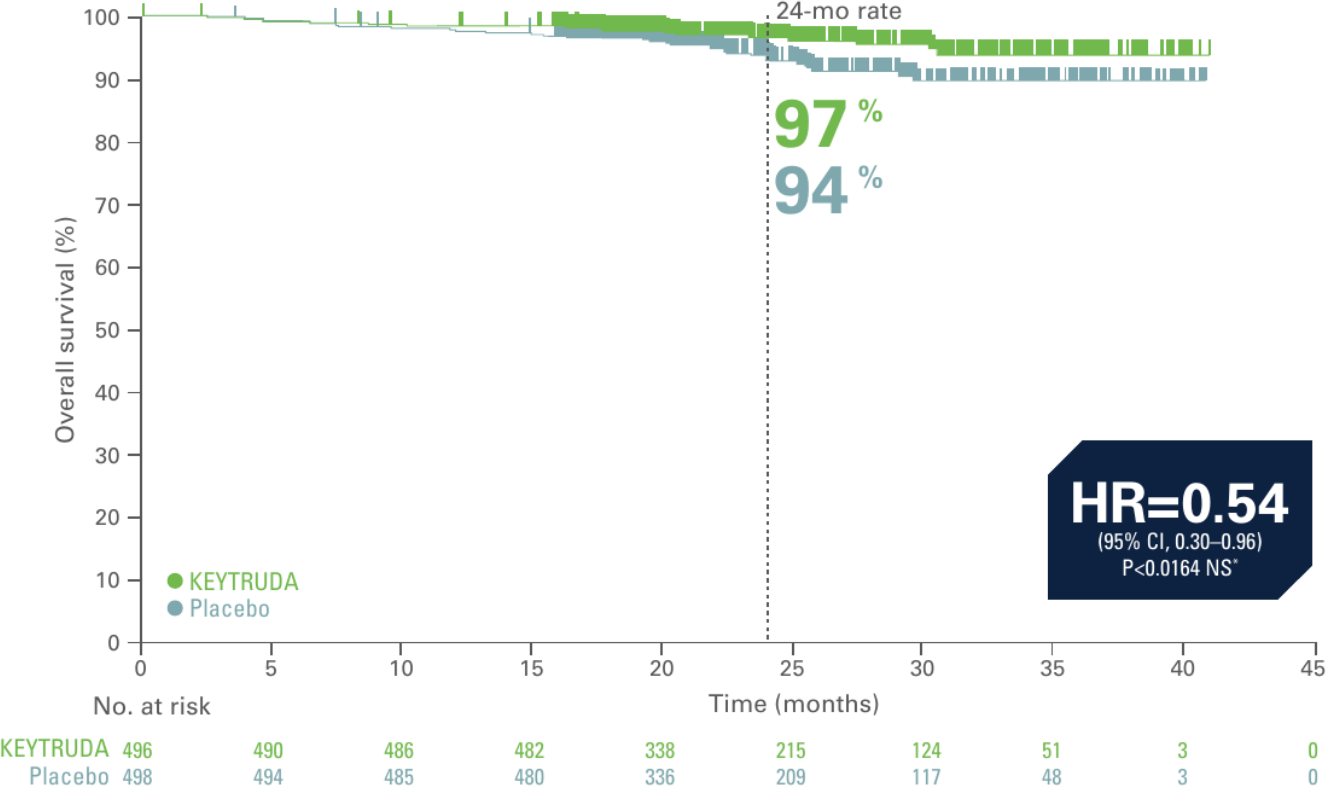
Adapted from Choueri TK et al. N Engl J Med. 2021;385(8):683–694.
- 46% reduction in the risk of death with KEYTRUDA vs. placebo2
- At the time of analysis, OS results were not yet mature, with 18 deaths out of 496 patients in the KEYTRUDA arm and 33 deaths out of 498 patients in the placebo arm2
Primary analysis data cutoff date: December 14, 2020.2
*Did not cross prespecified p-value boundary for statistical significance.
Kaplan-Meier estimate of overall survival in interim analysis of KEYNOTE-564 (secondary endpoint, ITT population, 30.1 month median follow-up)3
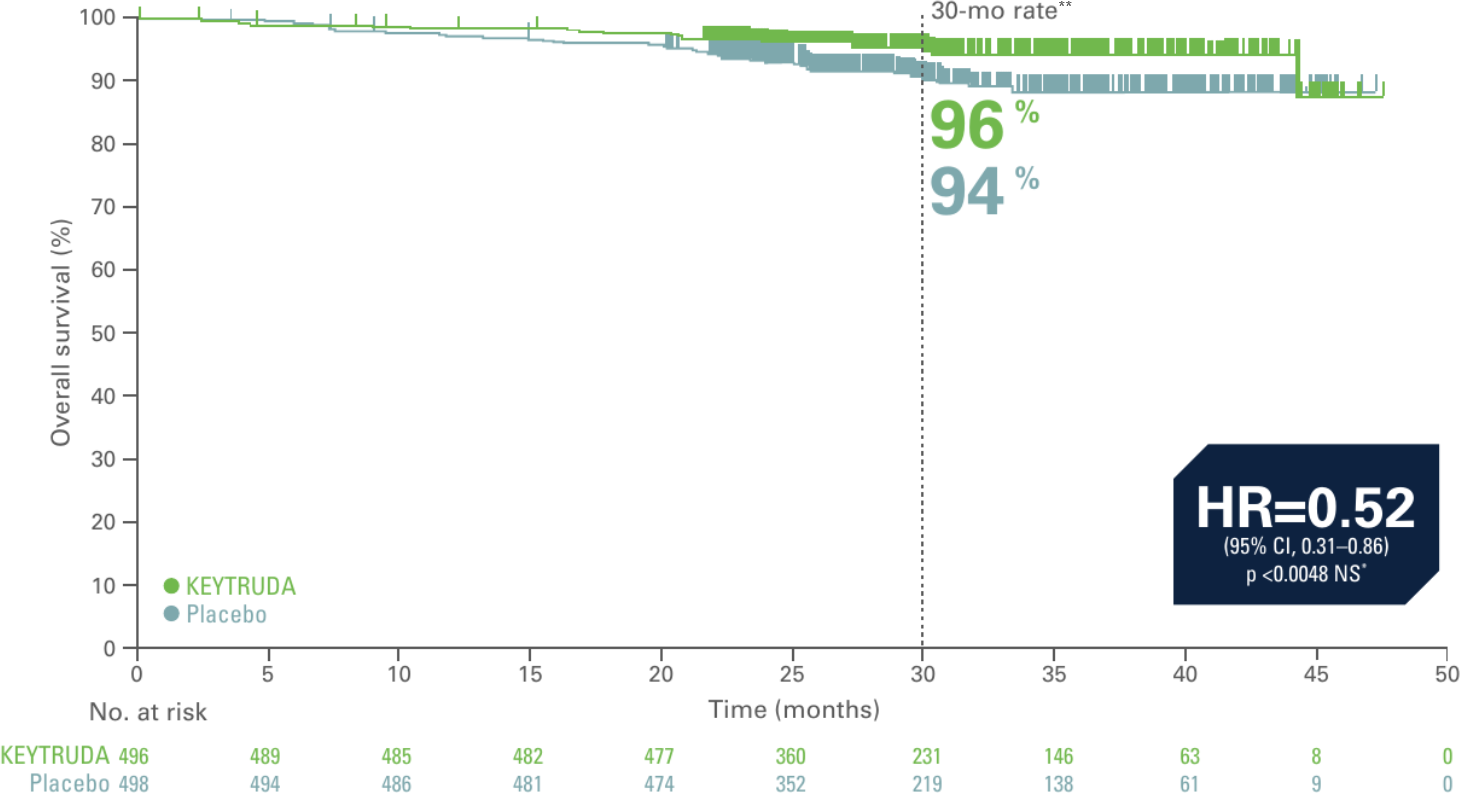
Adapted from Choueri TK et al. Presented at ASCO 2022. J Clin Oncol. 2022;40:6 suppl:290-290.3
**Time point was not part of the prespecified analysis and therefore a statistically significant conclusion cannot be drawn.
- 48% reduction in the risk of death with KEYTRUDA vs. placebo3
- Median OS has yet to be reached in both treatment arms3
- At the time of analysis, 23 patients in the KEYTRUDA group had an OS event compared to 43 patients in the placebo group. OS results were not yet mature, as only 33% of deaths needed for the final analysis had accrued at data cutoff3
Updated analysis data cutoff date: June 14, 2021.3
*Did not cross prespecified p-value boundary for statistical significance.3
Patients demonstrate a manageable safety profile2,3
Treatment related adverse events with an incidence of ≥5% in either group (as-treated population)4
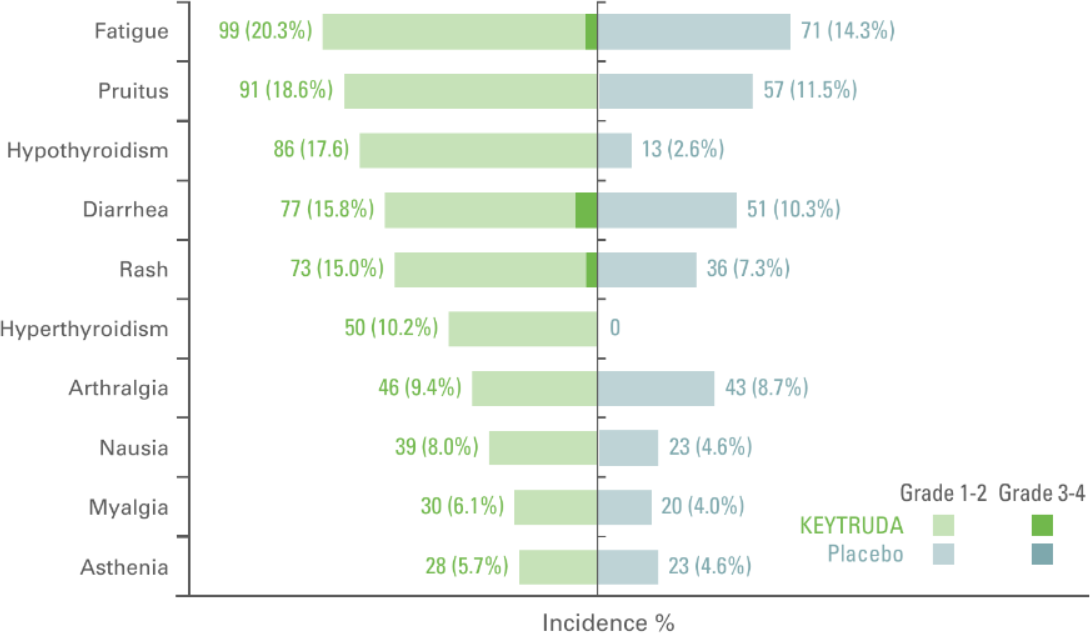
Adapted from Choueri TK et al. Presented at ASCO 2021 N Engl J Med. 2021;385(8):683–694.
- The median duration of exposure to KEYTRUDA was 11.1 months (0.0–14.3 months) vs. 11.1 months (0.0–15.4 months) with placebo2
- In the as-treated population, 96.3% of patients in the KEYTRUDA arm had AEs of any grade and of any cause vs. 91.1% in the placebo arm2
- In total, 32.5% of patients who received KEYTRUDA and 17.7% of those who received placebo had an AE of Grade 3 to 52
- 79.1% of patients had treatment-related AEs in the KEYTRUDA arm (18.9% of these patients had Grade 3 to 4) vs. 53.4% in the placebo arm (1.2% Grade 3 to 4)2
- In the as-treated population, 20.7% of patients in the KEYTRUDA arm vs. 2.0% in the placebo arm discontinued treatment due to AEs2
Primary analysis data cutoff date: December 14, 2020.2
Median follow up was 24.1 months in this analysis.2
*At the time of analysis, OS results were not yet mature, with 18 deaths out of 496 patients in the KEYTRUDA arm and 33 deaths out of 498 patients in the placebo arm.2
Treatment related adverse events with an incidence of ≥5% in either group (as-treated population)5
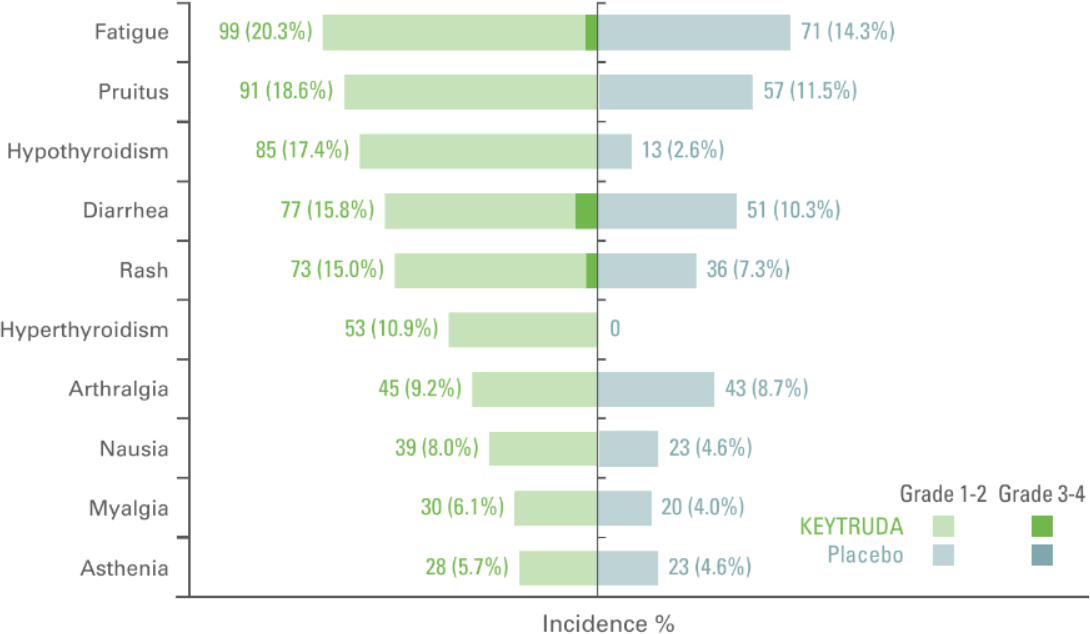
Adapted from Choueri TK et al. Presented at ASCO 2022. J Clin Oncol. 2022;40:6 suppl:290-290.
- The median duration of exposure to KEYTRUDA was 11.1 months (0.0–14.3 months) vs. 11.1 months (0.0–15.4 months) with placebo3
- In the as-treated population, 96.3% of patients in the KEYTRUDA arm had AEs of any grade and of any cause vs. 91.3% in the placebo arm3
- In total, 32.2% of patients who received KEYTRUDA and 17.7% of those who received placebo had an AE of Grade 3 to 53
- 79.1% of patients had treatment-related AEs in the KEYTRUDA arm (18.6% of these patients had Grade 3 to 4) vs. 53.4% in the placebo arm (1.2% Grade 3 to 4)3
- In the as-treated population, 21.1% of patients in the KEYTRUDA arm vs 2.2% in the placebo arm discontinued treatment due to AEs3
Updated analysis data cutoff date: June 14, 2021.3
Median follow up was 30.1 months in this analysis.3
Any Grade immune-related adverse events occurring in ≥1% of patients (as-treated population)6
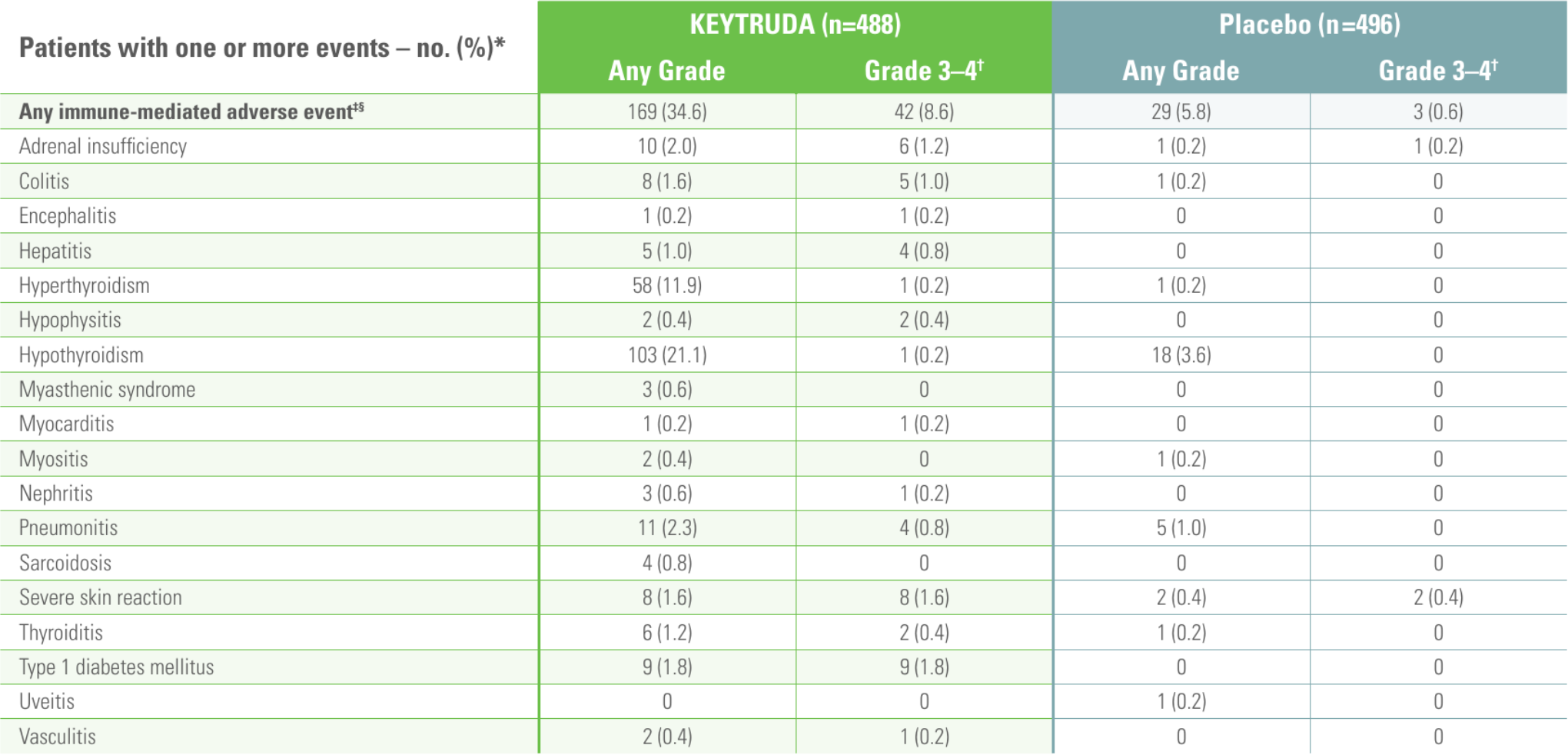
*The as-treated population was defined as all patients who received at least 1 dose of study drug treatment.6
†No Grade 5 immune-mediated adverse events occurred.6
‡Based on a prespecified list of terms included regardless of attribution to study treatment by investigator.6
§Infusion reactions of any Grade and Grade 3–4 occurred in 7 (1.4%) and 2 patients (0.4%), respectively, with KEYTRUDA and in 5 (1.0%) and no patients, respectively, with placebo.6

Connect with us
To share your thoughts on these data and arrange a meeting at your convenience, email us at: msdukoncology@msd.com
References
- KEYTRUDA Summary of Product Characteristics
- Choueri TK et al. N Engl J Med. 2021;385(8):683–694.
- Choueri TK et al. J Clin Oncol. 2022;40:6 suppl:290–290.
- Adapted from Choueri TK et al. Presented at ASCO 2021. N Engl J Med. 2021;385(8):683–694.
- Adapted from Choueri TK et al. Presented at ASCO 2022. J Clin Oncol. 2022;40:6 suppl:290-290.
- Choueri TK et al. N Engl J Med. 2021;385(8):683–694. Suppl.
Supporting documentation
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland)
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website
