Be alerted to new data & content
Be the first to see:
✔ The latest product updates
✔ Receive relevant supportive materials and resources
KEYTRUDA® (PEMBROLIZUMAB) IN MELANOMA
Bringing immunotherapy to Stage IIB-IV melanoma
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland) [External links]
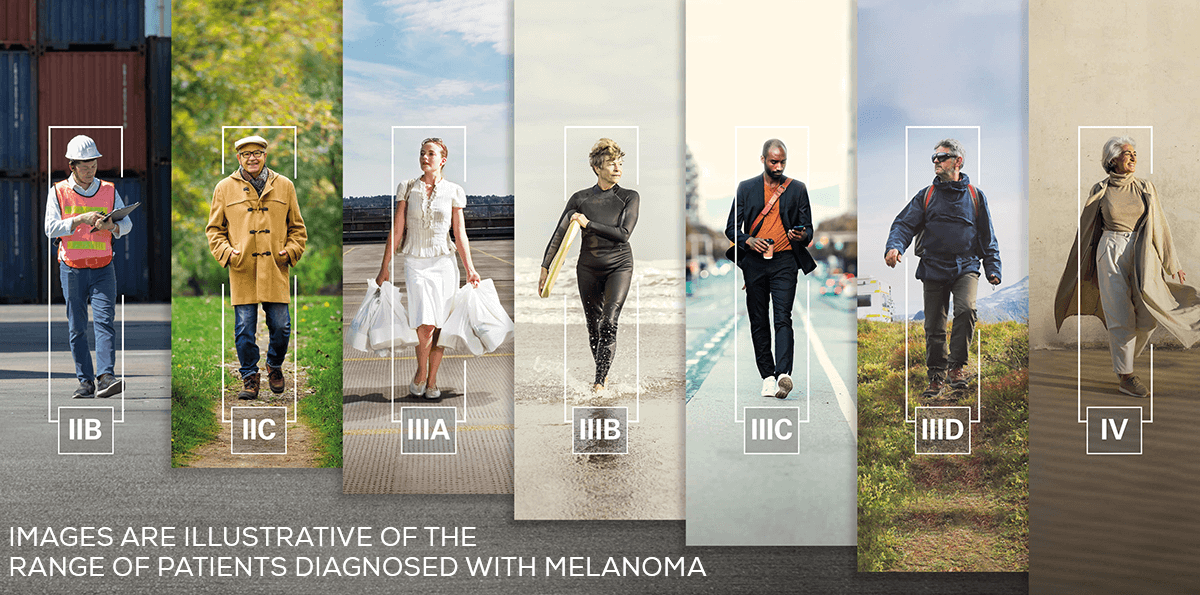
Resected
Stage IIB & IIC
melanoma
KEYTRUDA can be used in the adjuvant treatment of Stage IIB and IIC melanoma after complete resection
Resected
Stage III melanoma
KEYTRUDA can be used in the adjuvant treatment of Stage III melanoma after complete resection

Find out more about KEYTRUDA in Stage III melanoma
Advanced
melanoma
KEYTRUDA can be used in the treatment of advanced (unresectable or metastatic) melanoma

Find out more about KEYTRUDA in advanced melanoma (coming soon)
KEYTRUDA as monotherapy is indicated for:
Adult patients
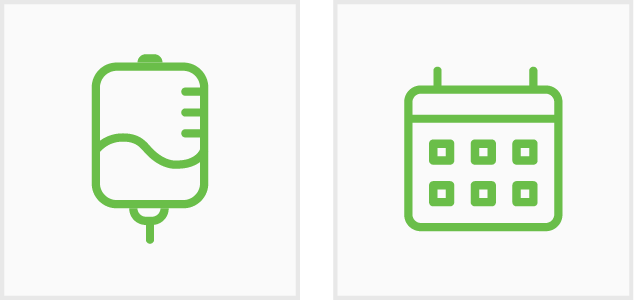
200 mg
every three weeks

400 mg
every six weeks
Paediatric patients

2 mg/kg bw
every three weeks, up to a maximum of 200 mg
30 minute
IV infusion time
For adjuvant treatment, patients should receive KEYTRUDA until disease recurrence, unacceptable toxicity or up to one year. For advanced melanoma, patients should receive KEYTRUDA until disease recurrence or unacceptable toxicity. Please refer to the SmPC before prescribing.
Be the first to see:
✔ The latest product updates
✔ Receive relevant supportive materials and resources

IV = Intravenous
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland)
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website
Loading...

This section of MSD Connect is an online portal containing promotional information about MSD pharmaceutical products, therapy area materials and professional resources and is intended for UK Healthcare Professionals.
To contact us please telephone 0208 154 8000 or email medicalinformationuk@msd.com | Privacy Policy | Terms of Use
GB-NON-07197 | Date of Preparation: August 2023
Merck Sharp & Dohme (UK) Limited Registered Office: 120 Moorgate, London, EC2M 6UR, United Kingdom.
Registered in England No. 233687 Copyright © 2023 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Merck Sharp & Dohme (UK) Limited (Tel: 0208 154 8000)
This section of the website contains promotional information intended for UK Healthcare Professionals only. If you are not a UK healthcare professional, please click here. GB‑NON‑07725 | Date of Preparation: August 2023