Be alerted to new data & content
Be the first to see:
✔ The latest product updates
✔ Receive relevant supportive materials and resources
KEYTRUDA® (PEMBROLIZUMAB) IN RESECTED STAGE IIB AND IIC MELANOMA
Moving from observation to action
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland) [External links]

KEYTRUDA as monotherapy is indicated for:
Did you know that patients with resected Stage IIB and IIC melanoma are still at high risk of recurrence?
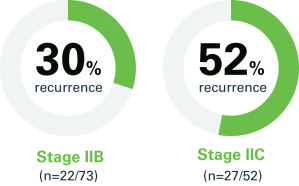
Relapse rates in patients with Stage IIB melanoma and Stage IIC melanoma were 32% and 46%, respectively*
Data from a US-based study shows recurrence rates in patients with Stage IIB–IIC melanoma range from 32–46%; of these, 30–52% recur with distant metastasis.1
Stage IIB and Stage IIC relapse rates1
Node Negative (MO)


After surgical resection of Stage IIB and IIC melanoma, patients are still at high risk of recurrence, with
half of relapses occurring within 2 years.
Stage IIB and Stage IIC median time to relapse1


Stage IIB and Stage IIC patients who relapsed with distant metastasis1
Percentage of patients who relapsed with distant metastasis as first relapse*
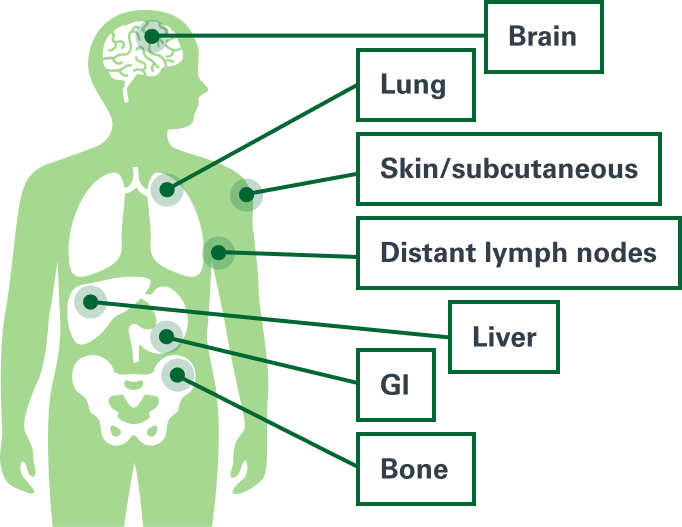
GI = Gastrointestinal
*The remainder of patients experienced a local/in-transit or regional nodal relapse.
A retrospective review of 738 adult patients from a prospectively maintained, single-institution database, with resected pathologic Stage II primary cutaneous melanoma (AJCC 7th ed.). All patients were treated at Memorial Sloan Kettering Cancer Center, USA, between January 1993 and December 2013. Patients underwent pathological nodal staging by sentinel lymph node biopsy or elective lymph node dissection. Median follow-up of patients with Stage IIB and Stage IIC melanoma was 52 months (50.2 and 46.2 months, respectively). Synchronous initial relapses were scored by the most advanced site. Secondary primary melanomas were not recorded as relapses.
Melanoma-specific survival rates at 5 and 10 years based on AJCC 8th edition pathologic melanoma staging criteria2
Survival data generated using IMDPP database, containing records of >46,000 patients with melanoma (n=43,792 qualified for analysis). Included patient records from 10 institutions in the US, Europe and Australia with melanoma at Stage I–III at initial diagnosis and had received treatment since 1998.
Staging was carried out according to AJCC 8th edition pathologic staging criteria for melanoma.
Reference: Gershenwald JE, et al. CA Cancer J Clin 2017;67:472–492.
KEYTRUDA is available as an adjuvant treatment option for
your eligible Stage IIB and IIC resected melanoma patients.3
The marketing authorisation was granted based on data from KEYNOTE-716, which was a multicentre, randomised, double-blind, placebo-controlled phase III trial in patients with completely resected Stage IIB or IIC melanoma. 976 patients were randomised to receive either KEYTRUDA (n = 487) Q3W or placebo (n = 489) Q3W for up to 1 year.4

If you want to learn more about KEYTRUDA in the adjuvant treatment of Stage IIB/C melanoma, download our clinical data presentation
Primary endpoint: Kaplan-Meier estimates of recurrence-free survival in the ITT population at the first interim analysis*†4

Adapted from: Luke JJ et al. Lancet 2022;399:1718–1729
*Recurrence-free survival was defined as the time from randomisation to first recurrence of melanoma at any site (local [recurrence in the immediate vicinity of the primary tumour], in-transit or regional [regional lymph node basin involvement], or distant [including events diagnosed within 30 days of a local, regional, or locoregional event]) or death due to any cause, whichever occurred first.
†Based on Cox regression model with Efron’s method of tie handling with treatment as a covariate stratified by melanoma T category (T3b vs. T4a vs. T4b). IA1 data cutoff: 04 December 2020.
In the first interim analysis, at a median follow up of 14.4 months, a HR of 0.65 demonstrated a 35% risk reduction in disease recurrence with KEYTRUDA vs. placebo.4
The safety profile of KEYTRUDA among the patients with resected melanoma enrolled in KEYNOTE‑716 was consistent with previous studies.4
Treatment-related adverse events at the first interim analysis*
| KEYTRUDA (n = 483) | KEYTRUDA (n = 483) | Placebo (n = 486) | Placebo (n = 486) | |
|---|---|---|---|---|
| Any | Grade ≥3 | Any | Grade ≥3 | |
| Any cause adverse event | 449 (93%) | 125 (26%) | 433 (89%) | 83 (17%) |
| Any treatment-related adverse event | 386 (80%) | 78 (16%) | 296 (61%) | 21 (4%) |
| Treatment-related events occurring in ≥5% of patients in each group | ||||
| Hypothyroidism | 70 (14%) | 0 | 12 (2%) | 0 |
| Hyperthyroidism | 48 (10%) | 1 (<1%) | 3 (1%) | 0 |
| Diarrhoea | 85 (18%) | 5 (1%) | 51 (10%) | 1 (<1%) |
| Nausea | 38 (8%) | 0 | 31 (6%) | 0 |
| Fatigue | 98 (20%) | 1 (<1%) | 87 (18%) | 0 |
| Asthenia | 43 (9%) | 1 (<1%) | 40 (8%) | 0 |
| Arthralgia | 69 (14%) | 1 (<1%) | 35 (7%) | 0 |
| Myalgia | 27 (6%) | 2 (<1%) | 14 (3%) | 0 |
| Increased alanine aminotransferase | 34 (7%) | 4 (1%) | 18 (4%) | 1 (<1%) |
| Increased aspartate aminotransferase | 28 (6%) | 1 (<1%) | 8 (2%) | 1 (<1%) |
| Pruritus | 112 (23%) | 3 (1%) | 48 (10%) | 0 |
| Rash | 75 (16%) | 7 (1%) | 29 (6%) | 1 (<1%) |
| Rash maculopapular | 34 (7%) | 2 (<1%) | 8 (2%) | 0 |
| Adverse events of interest† | ||||
| Hypothyroidism | 76 (16%) | 0 | 17 (3%) | 0 |
| Hyperthyroidism | 50 (10%) | 1 (<1%) | 3 (1%) | 0 |
| Colitis | 17 (4%) | 8 (2%) | 4 (1%) | 0 |
| Adrenal insufficiency | 11 (2%) | 4 (1%) | 0 | 0 |
| Hepatitis | 10 (2%) | 8 (2%) | 3 (1%) | 2 (<1%) |
| Hypophysitis | 10 (2%) | 3 (1%) | 0 | 0 |
| Infusion reactions | 5 (1%) | 0 | 6 (1%) | 0 |
| Myasthenic syndrome | 2 (<1%) | 2 (<1%) | 0 | 0 |
| Myelitis | 1 (<1%) | 1 (<1%) | 0 | 0 |
| Myocarditis | 0 | 0 | 1 (<1%) | 1 (<1%) |
| Myositis | 6 (1%) | 3 (1%) | 0 | 0 |
| Nephritis | 7 (1%) | 2 (<1%) | 0 | 0 |
| Pancreatitis | 2 (<1%) | 2 (<1%) | 0 | 0 |
| Pneumonitis | 9 (2%) | 1 (<1%) | 3 (1%) | 0 |
| Sarcoidosis | 5 (1%) | 0 | 0 | 0 |
| Severe skin reactions | 14 (3%) | 13 (3%) | 3 (1%) | 3 (1%) |
| Thyroiditis | 8 (2%) | 0 | 2 (<1%) | 0 |
| Type 1 diabetes | 2 (<1%) | 2 (<1%) | 0 | 0 |
| Uveitis | 1 (<1%) | 0 | 0 | 0 |
Adapted from: Luke JJ et al. Lancet 2022;399:1718-1729.
†Adverse events of interest (immune-mediated adverse events and infusion reactions) were based on a list of terms specified by the sponsor, regardless of attribution to any study treatment by investigators. All adverse events of interest are reported. Only the highest reported grade of a given adverse event is counted for an individual patient.
Summary of adverse events in KEYNOTE-716 at first interim analysis*
| Adverse event, n (%) | KEYTRUDA (n=483) | Placebo (n=486) |
|---|---|---|
| Any | 449 (93) | 433 (89) |
| Grade 3–5 | 125 (26) | 83 (17) |
| Led to discontinuation | 75 (16) | 20 (4) |
| Led to death | 0 (0) | 4 (0.8)† |
| Treatment-related | 386 (80) | 296 (61) |
| Grade 3–5 | 78 (16) | 21 (4) |
| Led to death | 0 (0) | 0 (0) |
Adapted from: Luke JJ et al. Lancet 2022;399:1718–1729
*The safety population included all patients who were randomly assigned to treatment and received at least one dose of study treatment.
†Four deaths occurred: one due to COVID-19-related pneumonia, one due to pneumonia, one due to recurrent cancer, and one due to suicide.
KEYTRUDA is associated with immune-mediated adverse events. Please refer to the Summary of Product Characteristics for full details about adverse events and their management.3

Adapted from: Long GV et al. Lancet Oncol 2022;23(11):1378–1388
IA3 data cutoff: January 04, 2022.
*DMFS was defined as the time from randomisation to the first diagnosis of distant metastasis.
†Based on Cox regression model with Efron’s method of tie handling with treatment as a covariate stratified by melanoma T category (T3b vs. T4a vs. T4b).
In the third interim analysis, at a median follow up of 27.4 months, a HR of 0.64 demonstrated a significant improvement in DMFS with KEYTRUDA vs. placebo.7
The safety profile of KEYTRUDA among patients with resected melanoma in the third interim analysis was similar to the initial analysis.7
These results are from an exploratory long-term analysis and should be interpreted with caution. Significance was not tested; therefore, no statistical conclusions can be drawn from this analysis

Adapted from: Luke JJ et al. ASCO 2023. Oral presentation
Final analysis data cutoff: January 04, 2023.
*Recurrence-free survival was defined as the time from randomisation to first recurrence of melanoma at any site (local [recurrence in the immediate vicinity of the primary tumour], in-transit or regional [regional lymph node basin involvement], or distant [including events diagnosed within 30 days of a local, regional, or locoregional event]) or death due to any cause, whichever occurred first.
†Based on Cox regression model with Efron’s method of tie handling with treatment as a covariate stratified by melanoma T category (T3b vs. T4a vs. T4b).
In the final analysis, at a median follow up of 39.4 months, adjuvant KEYTRUDA continued to demonstrate a risk reduction in disease recurrence vs. placebo.5,6
These results are from an exploratory long-term analysis and should be interpreted with caution. Significance was not tested; therefore, no statistical conclusions can be drawn from this analysis

Adapted from: Luke JJ et al. ASCO 2023. Oral presentation
Final analysis data cutoff: 04 January 2023.
*Based on Cox regression model with Efron’s method of tie handling with treatment as a covariate stratified by melanoma T category (T3b vs. T4a vs. T4b).
**DMFS was defined as the time from randomisation to the first diagnosis of distant metastasis.
In the final analysis, at a median follow up of 39.4 months, adjuvant KEYTRUDA demonstrated a continued improvement in DMFS vs. placebo.5,6
The safety profile of KEYTRUDA among the patients with resected melanoma in the final analysis of KEYNOTE-716 was consistent with previous analyses.4,7
Be the first to see:
✔ The latest product updates
✔ Receive relevant supportive materials and resources
AE = Adverse Event; CI = Confidence Interval; DMFS = Distant Metastasis-Free Survival; HR = Hazard Ratio; ITT = Intention-To-Treat; RFS = Recurrence-Free Survival; Q3W = Every 3 weeks; IA = Interim Analysis.
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland)
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website
Loading...

This section of MSD Connect is an online portal containing promotional information about MSD pharmaceutical products, therapy area materials and professional resources and is intended for UK Healthcare Professionals.
To contact us please telephone 0208 154 8000 or email medicalinformationuk@msd.com | Privacy Policy | Terms of Use
GB-NON-07197 | Date of Preparation: August 2023
Merck Sharp & Dohme (UK) Limited Registered Office: 120 Moorgate, London, EC2M 6UR, United Kingdom.
Registered in England No. 233687 Copyright © 2023 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Merck Sharp & Dohme (UK) Limited (Tel: 0208 154 8000)
This section of the website contains promotional information intended for UK Healthcare Professionals only. If you are not a UK healthcare professional, please click here. GB‑NON‑07725 | Date of Preparation: August 2023