KEYNOTE-407: KEYTRUDA plus chemotherapy in squamous mNSCLC
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland) [External links]
Discover the 5-year follow-up data from KEYNOTE-407: KEYTRUDA plus chemotherapy in squamous mNSCLC*
KEYNOTE-407 study design1
A randomised, multicentre, double-blind, placebo-controlled, phase III trial in patients (n=559) with previously untreated metastatic squamous NSCLC.1
559 patients were randomly assigned (in a 1:1 ratio) to receive 200 mg of pembrolizumab plus chemotherapy (carboplatin and either paclitaxel or nanoparticle albumin-bound [nab]– paclitaxel) or saline placebo plus chemotherapy (carboplatin and either paclitaxel or nabpaclitaxel) on Day 1 for up to 35 cycles.1
Overall survival and progression-free survival were primary endpoints. Secondary endpoints consisted of overall response rate, duration of response, and safety.1
The results of this phase III trial were first published with a median follow-up of 7.8 months.1
The latest follow-up was published with a median follow-up of 56.9 months.2
The original analysis1
KEYTRUDA plus carboplatin-paclitaxel/nab-paclitaxel provides superior survival vs. plat-pac/nabpac for the first-line treatment of metastatic squamous NSCLC in adults:1
- Superior OS with a 36% reduction in the risk of death (HR 0.64, p<0.001)
- Superior PFS with a 44% reduction in the risk of progression or death (HR 0.56, p<0.001)
- The overall survival benefit was consistent regardless of the level of PD-L1 expression*
- Superior ORR (57.9% vs. 38.4%) and improved DOR
- A generally manageable tolerability profile that is comparable to carb-pac/nabpac1
*Exploratory endpoints – no statistical conclusions can be drawn from them.
At a median follow-up of 56.9 months, KEYTRUDA plus chemotherapy (carboplatin-paclitaxel/nab-paclitaxel) continued to provide long-term survival benefit vs. Carboplatin-paclitaxel/nab-paclitaxel for the first-line treatment of metastatic squamous NSCLC in adults.2
5 year OS rate of
18.4%
KEYTRUDA + carbo + pac/nab-pac
vs.
9.7%
Carbo + pac/nab-pac
(HR 0.71, p not tested)
Exploratory endpoints – no statistical conclusions can be drawn from them
Median OS of
17.2 months
KEYTRUDA + carbo + pac/nab-pac
vs.
11.6 months
Carbo + pac/nab-pac
(p not tested)
Exploratory endpoints – no statistical conclusions can be drawn from them
Survival benefit observed in each of the PD-L1 TPS subgroups, including the PD-L1 TPS <1% expressers:2
22.1%
KEYTRUDA + carbo + pac/nab-pac
vs.
16.4%
Carbo + pac/nab-pac
(HR 0.78, p not tested)
Exploratory endpoints – no statistical conclusions can be drawn from them
Overall response rate of
62.6%
KEYTRUDA + carbo + pac/nab-pac
vs.
38.8%
Carbo + pac/nab-pac
Exploratory endpoints – no statistical conclusions can be drawn from them
Overall Survival (OS): KEYTRUDA + carbo + pac/nab-pac vs. carbo + pac/nab-pac3, a
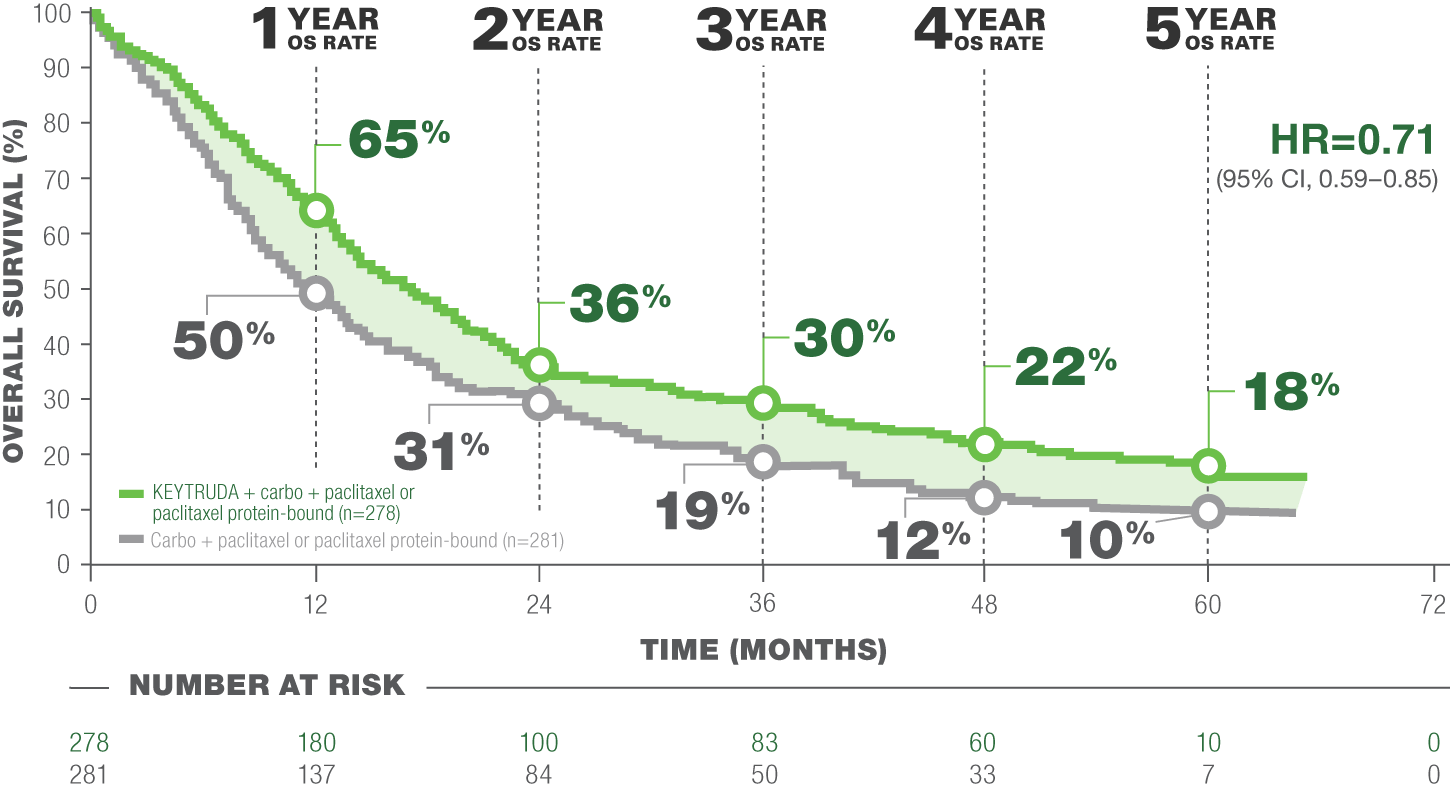
LIMITATION: This post hoc analysis (median follow-up time: 56.9 months ) in KEYNOTE-407 was exploratory in nature and occurred after the protocol-specified final analysis. No formal statistical testing was planned for this analysis and, therefore, no statistical conclusions can be drawn. Trial participants in either study arm could receive subsequent anti-cancer therapy.3
CROSSOVER RATE: 50.9; 117 patients crossed over to KEYTRUDA monotherapy on-study and an additional 26 received subsequent anti–PD-(L)1 therapy outside the study3,b
Median OS: 17.2 months (95% CI, 14.4–19.7) with KEYTRUDA + carbo + pac/nab-pac vs. 11.6 months (95% CI, 10.1–13.7) with carbo + pac/nab-pac3
Events: 80.9% (225/278) with KEYTRUDA + carbo + pac/nab-pac and 88.3% (248/281) with carbo + pac/nab-pac3
a Time from randomization to date of data cutoff.2
b anti–PD-L1 or anti–PD-1.
Adapted from “5-year update from KEYNOTE-407: pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer (NSCLC).” Novello S, Kowalski DM, Luft A, et al.
Summary of AEs in all treated patients (5-year update)3
Median follow up: 56.9 months
A generally manageable tolerability profile that is comparable to carb-pac/nabpac.1, 2, 4 The frequency of adverse events for combination was comparable to previous studies.
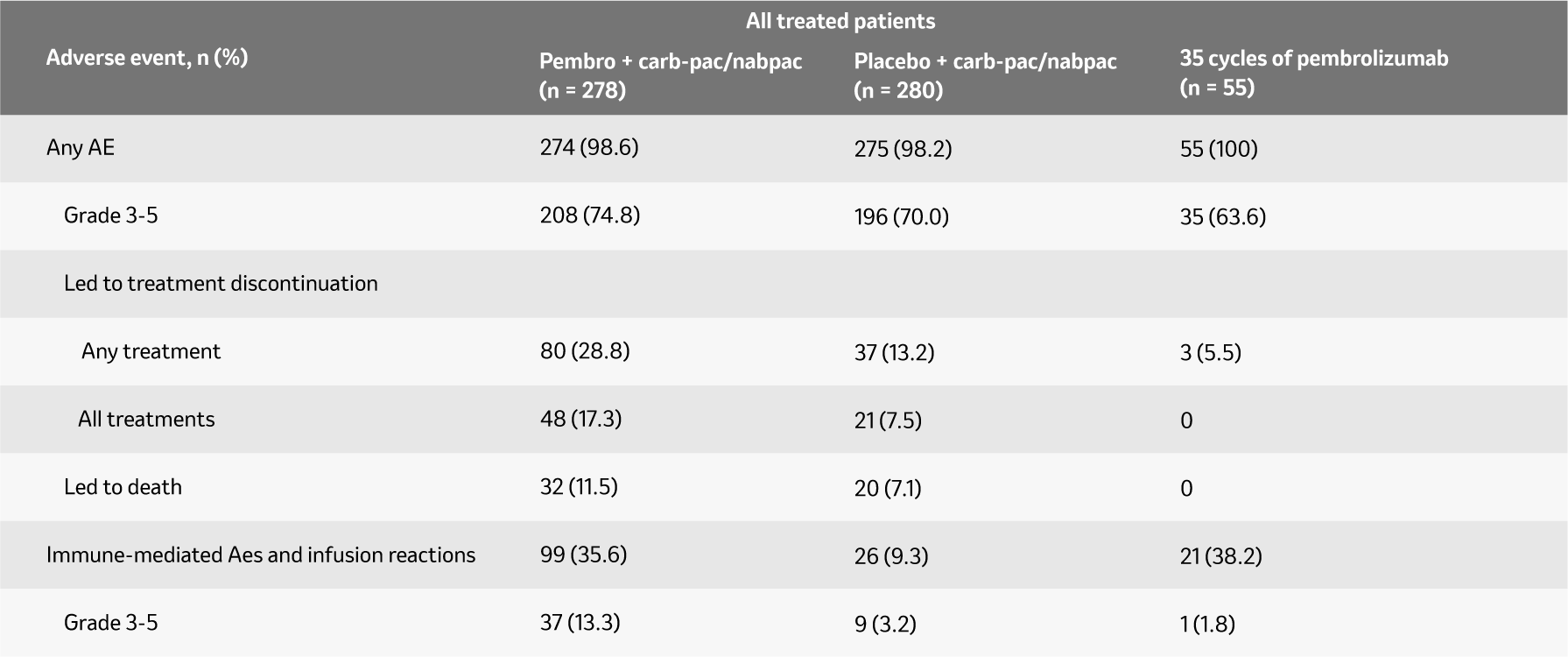
Adapted from: Novello S et al. ESMO 2022.
Analysis cut-off date: 23 February 2022.
a Includes patients who discontinued pembrolizumab or placebo, carboplatin, and taxane owing to an AE at any time and patients who discontinued pembrolizumab or placebo owing to an AE after completing four 3-week cycles of carboplatin and taxane.
b Events considered regardless of attribution to treatment or immune relatedness by the investigator.
c There was 1 Grade 3 event of colitis; there were no Grade 4 or Grade 5 immune-mediated AEs or infusion reactions among patients who completed 35 cycles of pembrolizumab.
Explore our related resources
Pembrolizumab with carboplatin and paclitaxel is recommended as an option for untreated metastatic squamous non-small-cell lung cancer (NSCLC) in adults, only if:5
- tumour proportion score of 0% to 49%
- tumour proportion score of 50% or more and they need urgent clinical intervention
- it is stopped at 2 years of uninterrupted treatment or earlier if their disease progresses and
- the company provides pembrolizumab according to the commercial arrangement
Pembrolizumab is accepted for restricted use within NHS Scotland in combination with carboplatin and either paclitaxel or nabpaclitaxel, for the first-line treatment of metastatic squamous non-small cell lung cancer (NSCLC) in adults whose tumours express programmed death ligand 1 (PD-L1) with a <50% tumour proportion score (TPS), or in those whom it has not been possible to evaluate PD-L1 TPS.6
KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous NSCLC in adults.4
The recommended dose of KEYTRUDA as part of combination therapy is either 200 mg Q3W or 400 mg Q6W administered intravenously over 30 minutes. KEYTRUDA should be administered first when given in combination with chemotherapy.4
Find out more about KEYTRUDA in mNSCLC
KEYTRUDA in combination with Chemotherapy in non-squamous mNSCLC
Monotherapy in mNSCLC
Patient Management
Sign up to MSD emails
✔ Get the latest product updates
✔ Receive cancer resources
✔ Be the first to hear about our events
Abbreviations:
carbo + pac/nab-pac = Carboplatin + Paclitaxel or Nab-Paclitaxel; CI = Confidence Interval; DOR = Duration of Response; HR = Hazard Ratio; mNSCLC = Metastatic Non–Small Cell Lung Carcinoma; NA = Not Available; ORR = Overall Response Rate; OS = Overall Survival; PD-L1 = Programmed Death Ligand 1; PFS = Progression Free Survival; TPS = Tumor Proportion Score; Q3W = Every Three Weeks; Q6W = Every Six Weeks
References
- Paz-Ares L et al. N Engl J Med. 2018; 379: 2040-2051.
- Robinson AG et al. Presented at the European Lung Cancer Virtual Congress (ELCC) 2021, 25–27 March 2021.
- Novello S, Kowalski DM, Luft A, et al. 5-year update from KEYNOTE-407: pembrolizumab plus chemotherapy in squamous non‒small-cell lung cancer. Slide deck presented at: European Society for Medical Oncology (ESMO) European Lung Cancer Virtual Congress (ELCC); 9–13 September 2022; Paris, France.
- KEYTRUDA Summary of Product Characteristics.
- National Institute for Health and Care Excellence (2022). Pembrolizumab with carboplatin and paclitaxel for untreated metastatic squamous non-small-cell lung cancer. Available from: https://www.nice.org.uk/guidance/ta770. NICE guidance is prepared for the National Health Service in England, and is subject to regular review and may be updated or withdrawn. NICE has not checked the user of its content in this document to confirm that it accurately reflects the NICE publication from with it is taken.
- Scottish Medicines Consortium (2019). Appraisal determination: Pembrolizumab 25mg/mL concentrate for solution for infusion and 50mg powder for concentrate for solution for infusion (KEYTRUDA®). Available from: https://www.scottishmedicines.org.uk/medicines-advice/pembrolizumab-keytruda-full-smc2187/.
Supporting documentation
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland)
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website


