About ZEPATIER
About ZEPATIER®▼ (elbasvir and grazoprevir)
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland) [External links]
SIMPLE* POWER TO CURE** FOR CHRONIC HEPATITIS C1-9

ZEPATIER is indicated for the treatment of chronic hepatitis C genotypes 1 and 4 infection in adults1
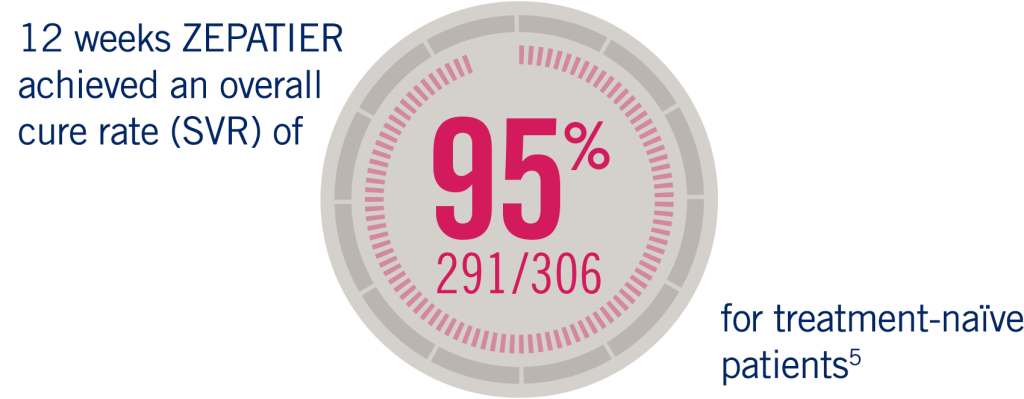
The C-EDGE Treatment Naïve study was an international, randomised, blinded, placebo-controlled, parallel-group trial of a fixed-dose combination of grazoprevir 100 mg/elbasvir 50 mg for treatment-naïve cirrhotic and non-cirrhotic patients with chronic HCV GT1, GT4, or GT6 infections. A historical SVR12 rate was used as the comparator for efficacy.
*‘Simple’ is supported by the information held on the DDIs and dosing simplicity page.
**Cure of HCV infection = sustained virologic response (SVR), the primary endpoint in all studies, was defined as hepatitis C virus (HCV) ribonucleic acid (RNA) less than the lower limit of quantification (LLOQ) (=15 IU/ml) at 12 weeks after the cessation of treatment.1
References
- ZEPATIER summary of Product Characteristics.
- Dore GL et al, Ann Int Med. 2016;doi:10.7326/M16-0816 [Epub ahead of print].
- Roth D et al. Lancet. 2015:386:1537-1545.
- Rockstroh JK et al. Lancet HIV. 2015:2(8);319-327.
- Zeuzem S et al. Ann Intern Med. 2015; 163:1-13.
- Forns X et al. J Hepatol. 2015;63:564-572.
- Kwo PY et al. C-EDGE TE EASL 2015.
- Sperl J et al. Presented at EASL April 13-17 2016; Barcelona Spain: Abstract PS002.
- Jacobson IM et al. Presented at: 66th Annual Meeting of the American Association for the Study of Liver Diseases; November 13-17 2015; Boston, MA.
- Europena Association for the Study of the Liver. J Hepatol. 2015;63:199-236.
Supporting documentation
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland)
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website