About doravirine
About doravirine▼
DELSTRIGO®▼ (doravirine/3TC/TDF)
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland) [External links]
PIFELTRO®▼ (doravirine)
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland) [External links]
Doravirine/3TC/TDF = DELSTRIGO (doravirine/lamivudine/tenofovir disoproxil fumarate).
What is doravirine?
Doravirine is an NNRTI that has demonstrated efficacy in both treatment-naïve and virologically supressed patients
PIFELTRO®▼ (doravirine) 100 mg film-coated tablet is indicated, in combination with other antiretroviral medicinal products, for the treatment of adults, and adolescents aged 12 years and older, weighing at least 35 kg, infected with HIV-1, without past or present evidence of resistance to the NNRTI class.
DELSTRIGO®▼ (100 mg doravirine/300 mg lamivudine/300 mg tenofovir disoproxil fumarate, equivalent to 245 mg tenofovir disoproxil) is indicated for the treatment of adults infected with HIV-1 without past or present evidence of resistance to the NNRTI class, lamivudine, or tenofovir.
DELSTRIGO®▼ is also indicated for the treatment of adolescents aged 12 years and older weighing at least 35 kg, who are infected with HIV-1, without past or present evidence of resistance to the NNRTI class, lamivudine, or tenofovir and who have experienced toxicities which preclude the use of other regimens that do not contain tenofovir disoproxil.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
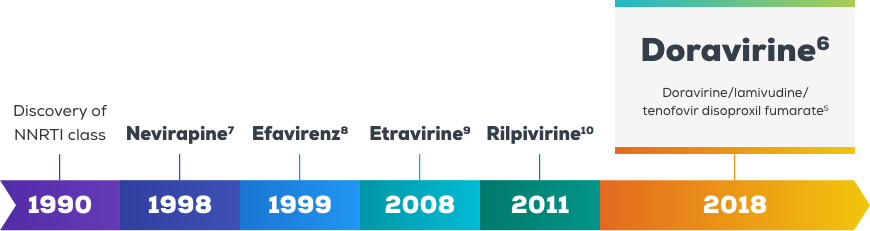
Doravirine – a next generation NNRTI1,2
See how doravirine can be a part of every day

Having an undetectable viral load supports U=U (undectable =Untransmittable) a global UNAIDS campaign supporting people living with HIV.
De Novo = Newly Acquired; NNRTI = Non-Nucleoside Reverse Transcriptase Inhibitor.
References
- Colombier M-A and Molina J-M. Doravirine: a review. Curr Opin HIV AIDS. 2018;13:308–314.
- Feng M, Sachs NA, Xu M, et al. Doravirine suppresses common nonnucleoside reverse transcriptase inhibitor-associated mutants at clinically relevant concentrations. Antimicrob Agents Chemother. 2016;60:2241–2247.
- Orkin C, Squires KE, Molina JM, et al.; and DRIVE-AHEAD Study Group. Doravirine/lamivudine/ tenofovir disoproxil fumarate is non-inferior to efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive adults with human immunodeficiency virus–1 infection: week 48 results of the DRIVE-AHEAD trial. Clin Infect Dis. 2019;68(4):535–544.
- Molina JM, Squires K, Sax PE, et al.; for the DRIVE-FORWARD Study Group. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV. 2018;5(5):e211– e220. doi:10.1016/S2352-3018(18)30021-3. Epub. 2018 March 25.
- DELSTRIGO Summary of Product Characteristics.
- PIFELTRO Summary of Product Characteristics.
- European Medicines Agency. EPAR summary for the public: nevirapine.
- European Medicines Agency. EPAR summary for the public: efavirenz.
- European Medicines Agency. EPAR summary for the public: etravirine.
- European Medicines Agency. EPAR summary for the public: rilpivirine.
Supporting documentation
DELSTRIGO®▼ Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland)
PIFELTRO®▼ Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland)
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website
