KEYTRUDA dosing recommendation:
Either 200 mg Q3W* or 400 mg Q6W** in monotherapy or combination approved indications for adults patients, administered intravenously over 30 minutes.
*Q3W: Every 3 weeks **Q6W: Every 6 weeks
UK Prescribing Information can be found here. [External link]
First-line indications
KEYTRUDA, in combination with platinum-containing chemotherapy as neoadjuvant treatment, then monotherapy as adjuvant treatment for resectable non-small cell lung carcinoma NSCLC at high risk of recurrence in adults.
KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous non-small cell lung carcinoma (NSCLC) in adults.
KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous non-small cell lung carcinoma (NSCLC) in adults whose tumours have no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) positive mutations.
KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic non-small cell lung carcinoma (NSCLC) in adults whose tumours express PD-L1 with a ≥50% tumour proportion score (TPS) with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) positive tumour mutations.
KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with non-small cell lung carcinoma who are at high risk of recurrence following complete resection and platinum based chemotherapy.
Previously treated indication
KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic non-small cell lung carcinoma (NSCLC) in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA.
KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with renal cell carcinoma at increased risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions.
KEYTRUDA, in combination with lenvatinib, is indicated for the first-line treatment of advanced renal cell carcinoma (aRCC) in adults.
KEYTRUDA is indicated in combination with axitinib for the first-line treatment of advanced renal cell carcinoma (aRCC) in adults.
KEYTRUDA as monotherapy is indicated for the treatment of adults and adolescents aged 12 years and older with advanced (unresectable or metastatic) melanoma.
KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults and adolescents aged 12 years and older with Stage IIB, IIC or III melanoma and who have undergone complete resection.
KEYTRUDA, in combination with chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment after surgery, is indicated for the treatment of adults with locally advanced, or early-stage triple-negative breast cancer at high risk of recurrence.
KEYTRUDA, in combination with chemotherapy, is indicated for the treatment of locally recurrent unresectable or metastatic triple-negative breast cancer in adults whose tumours express PD-L1 with a CPS ≥10 and who have not received prior chemotherapy for metastatic disease.
KEYTRUDA, in combination with carboplatin and paclitaxel, is indicated for the first-line treatment of primary advanced or recurrent endometrial carcinoma in adults.
KEYTRUDA, in combination with lenvatinib, is indicated for the treatment of advanced or recurrent endometrial carcinoma in adults who have disease progression on or following prior treatment with a platinum-containing therapy in any setting, and who are not candidates for curative surgery or radiation.
KEYTRUDA as monotherapy is indicated for adults with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer in the following settings:
KEYTRUDA, in combination with platinum and fluoropyrimidine-based chemotherapy, is indicated for the first-line treatment of locally advanced unresectable or metastatic carcinoma of the oesophagus in adults whose tumours express PD-L1 with a CPS ≥ 10.
KEYTRUDA, in combination with chemotherapy with or without bevacizumab, is indicated for the treatment of persistent, recurrent, or metastatic cervical cancer in adults whose tumours express PD‑L1 with a CPS ≥ 1
KEYTRUDA, as monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy, is indicated for the first-line treatment of metastatic or unresectable recurrent (M/uR) head and neck squamous cell carcinoma in adults whose tumours express PD-L1 with a CPS ≥1.
KEYTRUDA as monotherapy is indicated for the treatment of recurrent or metastatic head and neck squamous cell carcinoma in adults whose tumours express PD-L1 with a ≥50% TPS and progressing on or after platinum-containing chemotherapy.
KEYTRUDA, in combination with trastuzumab, fluoropyrimidine and platinum-containing chemotherapy, is indicated for the first-line treatment of locally advanced unresectable or metastatic HER2-positive gastric or gastro-oesophageal junction adenocarcinoma in adults whose tumours express PD-L1 with a CPS ≥ 1.
KEYTRUDA, in combination with fluoropyrimidine and platinum-containing chemotherapy, is indicated for the first-line treatment of locally advanced unresectable or metastatic HER2-negative gastric or gastro-oesophageal junction adenocarcinoma in adults whose tumours express PD-L1 with a CPS ≥ 1.
KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic urothelial carcinoma in adults who have received prior platinum-containing chemotherapy.
KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic urothelial carcinoma in adults who are not eligible for cisplatin-containing chemotherapy and whose tumours express PD-L1 with a combined positive score (CPS) ≥10.
KEYTRUDA, in combination with enfortumab vedotin, is indicated for the first-line treatment of unresectable or metastatic urothelial carcinoma in adults
KEYTRUDA as monotherapy is indicated for the treatment of adult and paediatric patients aged 3 years and older with relapsed or refractory classical Hodgkin lymphoma who have failed autologous stem cell transplant (ASCT), or following at least two prior therapies when ASCT is not a treatment option.
KEYTRUDA as monotherapy is indicated for the treatment of the following microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) tumours in adults with:
Either 200 mg Q3W* or 400 mg Q6W** in monotherapy or combination approved indications for adults patients, administered intravenously over 30 minutes.
*Q3W: Every 3 weeks **Q6W: Every 6 weeks
Please refer to the KEYTRUDA Summary of Product Characteristics and Risk Minimisation Materials before prescribing KEYTRUDA. Risk Minimisation Materials are available online, from your MSD representative or from MSD Medical Information (Email: medicalinformationuk@msd.com, Phone: 0208 154 8000).
KEYTRUDA is a selective monoclonal antibody that blocks the programmed cell death-1 (PD-1) protein pathway, potentiating T-cell responses, including anti-tumour responses.1,2
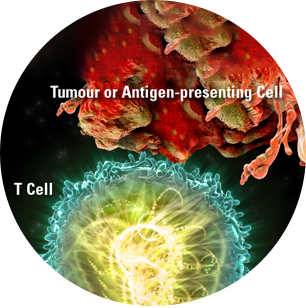
When functioning properly, T cells are activated and can attack tumour cells1,2
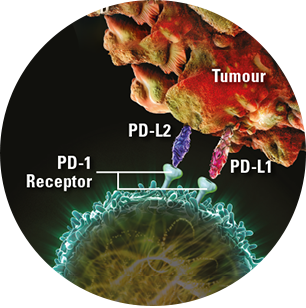
Some tumours can evade the immune system through the PD-1 pathway. On the surface of tumour cells the dual PD-1 ligands, PD-L1 and PD-L2, bind to the PD-1 receptors on T cells to inactivate them, allowing tumour cells to evade detection1,2
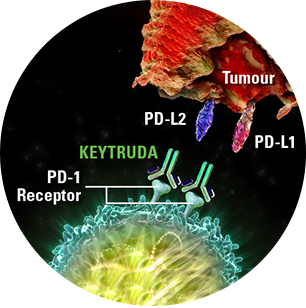
By inhibiting this process, KEYTRUDA reactivates tumour-specific cytotoxic T lymphocytes and anti-tumour immunity1,2
PD-1 = Programmed Cell Death-1; PD-L1 = Programmed Cell Death Ligand-1; PD-L2 = Programmed Cell Death Ligand-2.
UK Prescribing information can be found here. [External link]
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website.
This section of the website contains promotional information intended for UK Healthcare Professionals only. If you are not a UK healthcare professional, please click here.
GB‑NON‑10777 | Date of Preparation: January 2025

This section of MSD Connect is an online portal containing promotional information about MSD pharmaceutical products, therapy area materials and professional resources and is intended for UK Healthcare Professionals.
To contact us please telephone 0208 154 8000 or email medicalinformationuk@msd.com | Privacy Policy | Terms of Use
GB-NON-10696 | Date of Preparation: January 2025
Merck Sharp & Dohme (UK) Limited Registered Office: 120 Moorgate, London, EC2M 6UR, United Kingdom.
Registered in England No. 233687 Copyright © 2025 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Merck Sharp & Dohme (UK) Limited (Tel: 0208 154 8000)